Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
ACUTE RENAL FAILURE WITH NORMAL PLASMA UREA LEVELS: a Marker of Proximal Tubular Disfunction with Diabetes Insipidus
Case report
Musso CG1, 2, Giordani C1, Stonski E2, Peralta M2,
Bonetto A2, Jauregui R2, Algranati L1
1 Nephrology Department & 2Centro Agustin Rocca
Hospital Italiano de Buenos Aires-Argentina
Rev Electron Biomed / Electron J Biomed 2004;2:65-68
Comment Reviewer Dr Omar Abboud. Consultant Nephrologist, Hamad Medical Corporation, Doha, Qatar.
Comment Reviewer Jesús Garrido MD. Unidade de Nefrologia e Diálise. Hospital São Teotónio de Viseu. Viseu. Portugal.
Approximately 8% to 26% of patients who receive an aminoglycoside for more than several days will develop mild renal impairment that is almost always reversible. The toxicity results from accumulation and retetion of these drugs in the proximal tubular cells1.
In the following case report we present a particular pattern of acute renal failure documented in a patient treated with the aminoglycoside amikacin.
CASE REPORT
A 23 year-old woman was referred to our Nephrology Department presenting elevated serum creatinine levels in the context of normal serum urea.
Three month before admission she had suffered a fracture of her first and fourth lumbar vertebrae as a consequence of a car crash. In order to stabilize her spinal column, she had had to undergo an operation. Unfortunately she had developed a wound abscess. It was then when she was referred to our hospital where she had to be operated again. Bone samples were obtained and they confirmed the presence of an acute osteomyelitis secondary to Pseudomona Aeruginosa and metil-resistance Staphylococcus Aureus. Intravenous vancomicin 2 g/day, amikacin 1 g/day, piperacilin 16 g/day were prescribed.
After a month of treatment with this antimicrobial schedule an increase in her plasma creatinine level was detected: 1.2 mg/dl (initial value: 0.9 mg/dl). This acute renal failure was a non-oligoanuric one: diuresis: 4000 cc/day, and with low osmolality urine: 210 mosm/l. The antibiotic schedule was changed to a non nephrotoxic one and daily blood samples were taken to check urea and creatinine plasma levels. The patient had neither liver disease nor a low-protein diet. Her nutritional parameters were within the normal range, with a height and weight of 1.64 m and 61 kg respectively.
We appreciated a progressive increase in plasma creatinine levels without a significant alteration in plasma urea ones. Even more, the lowest plasma urea level (22 mg/dl) was detected when the highest plasma creatinine level (2.6 mg/dl) was documented (Table I).
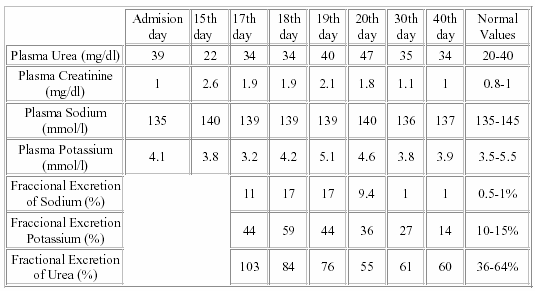
Aiming at explaining this phenomenon we measured urea and creatinine in blood and urine samples in order to calculate the fractional excretion of urea (FEU). As we can appreciate in Table I, fractional excretion of urea was excessively high (FEU: 103%), meaning that this substance was markely excreted, phenomenon that could explain the paradoxical normal plasma urea level in the context of an acute renal failure.
Since nephrotoxic drugs were removed, the acute renal failure progressively healed normalizing plasma creatinine values.
DISCUSSION
The acute renal failure previously described could be caused by two potential mechanisms: bone infection and nephrotoxic antibiotics. However, since renal deterioration begun after these drugs were iniciated and their remotion led to a clear renal function recovery we considered antibiotics as the main cause of this disturbance. Even though the three antibiotics prescribed in this case are potentially nephrotoxic2. Amikacin, an aminoglycoside, is the most nephrotoxic of them3.
Aminoglycosides undergoes renal excretion by glomerular filtration, although a small percentage of filtered drug is reabsorbed proximally. This reabsorbtion is done by pinocytosis through apical surface of tubular epithelial cells causing later perturbation of mitochondrial integrity and disruption of lysosomes into the proximal tubular cells4, 5. Aminoglycosides toxicity is mediated by necrotic and non-necrotic (apoptotic) mechanisms5. Parenchimal accumulation within the renal cortex occurs with these agents with tissue concentrations exceeding serum levels several fold, and on this tissue accumulation is based aminoglycoside proximal tubule toxicity3. However, dissociation between tissue accumulation and preservation of renal function has been reported4.
Aminoglycosides may alter urinary concentratation mechanisms and also reduce collecting tubule response to antidiuretic hormone3. The previous described statements could explain the low-osmolality polyuria documented in our patient.
The striking point of this case is the presence of normal plasma urea level in a context of renal insufficiency. In this case it could not be explained by the existence of a low muscle mass as she was neither malnourished nor small built or old.
In normal physiologic conditions, about 50% of the filtered urea is invariably reabsorbed in the proximal convoluted tubule. Some urea must also be reabsorbe in part of the distal nephron located in the cortex. Given the high cortical blood flow and the vascular-tubular organization in this area of the kidney, it seems highly unlike that any of this urea could be reintroduced in the nephron lumen. Thus, at least 50% of the filtered urea cannot be excreted. Because of that fractional excretion of urea higher than 50% could means urea reabsortion alteration or urea secretion. Since most of urea reabsortion is done in proximal tubules, area damaged by amikacin, a reduced proximal urea reabsorbtion could explain part of the high FEU found in this case6, 7, 8. The concomitantely elevated fractional excretion of sodium and potassium reinforce the hypothesis of an alteration of tubular reabsorbtion mechanisms.
Besides, since high urinary rate is another cause of increased urea excretion, the documented water diuresis in our patient, secondary to nephrogenic diabetes insipidus, could be another reason of her high FEU6.
Regarding creatinine, its high plasma levels could be not only explained by its reduced filtration (acute renal failure), but also by an alteration in its secretion due to amikacin-induced proximal toxicity.
CONCLUSION
Acute renal failure with normal urea plasma levels, in case of a young properly nourished patient, could be a marker of proximal tubular disfunction with diabetes insipidus.
REFERENCES:
-
1.- Chambers H. The aminoglycosides. In Goodman Gilman A, Limbird LE, Hardman JG (Eds). The pharmacological basis of therapeutic. New York. Medical Publishing Division. 2002: 1229
2.- Lacy C, Armstrong L, Goldman M, Lance L. Drug information handbook Hudson. Lexi-Comp; 2003: 1115-1116.
3.- Ahijado FJ, Garcia S. Nephrotoxic Acute Renal Failure. In Liaño F, Pascual J. (Eds). "Acute renal failure". Barcelona. Masson.2000: 139-170.
4.- Swan S, Bennett W. Nephrotoxic acute renal failure. In Lazarus JM, Brenner BM (Eds).Acute renal failure. New York. Curchill Livingstone. 1993: 361-4.
5.- El Mouedden M, Laurent G, Mingeot-Leclercq MP, Taper H, Cumps J, Tulkens. Apoptosis in renal proximal tubules of rats treated with low dosis of aminoglycosides. Antimicrobial Agents and Chemotherapy. 2000; 44: 665-675.
6.- Bankir L, Tinh-Trang-Tan MM. Urea and the kidney. Philadelphia. W. B. Saunders Company. 2000: 637-652.
7.- Kokko J. Overview of renal physiology. In Kokko J, Tannen R (eds). Fluids and electrolytes. Philadelphia. W.B Saunders Company. 1996: 855-856
8.- Kaplan A, Kohn O. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol 1992; 12: 49-54
RESUMEN
Aproximadamente 8% a 26% de los pacientes que reciben un aminoglucósido por un período mayor a varios días, desarrollan una insuficiencia renal leve que suele ser casi siempre reversible.
En este reporte describimos el caso una paciente mujer de 23 años en tratamiento con amikacina que presentó una insuficiencia renal aguda con poliuria acuosa, excreción fraccional de urea elevada y uremia normal, sin justificarse esto último por la presencia de hepatopatía, desnutrición, contextura corporal pequeña ni dieta vegetariana. Los aminoglucósidos pueden alterar el funcionamiento de los túbulos proximales, los mecanismos de concentración urinaria y la respuesta del nefrón distal a la vasopresina. Esto último podría explicar la presencia de una uremia normal en el contexto de una insuficiencia renal aguda, como el resultado de una marcada excreción urinaria de urea. La insuficiencia renal aguda con uremia normal, en paciente joven y no desnutrido, podría ser un marcador de disfunción tubular proximal con diabetes insípida.
Comment Reviewer Dr Omar Abboud. Consultant Nephrologist. Hamad Medical Corporation, Doha, Qatar.
Over all, the article is well presented. The proposed mechanism to explain the normal urea in the face of an elevated serum creatinine in a patient with acute renal failure is logical, and reasonably supported by the evidence given.
There are a few comments, below, to be taken in consideration:
Two potential mechanisms were proposed for the acute renal failure: infection and nephrotoxic antibiotics. It was assumed that drugs were the cause as the renal failure started after initiation of therapy and resolved after discontinuation of the drugs. Although I agree with the assumption, infection could have been the cause: leading to renal failure which resolved with the clearance of the infection with antibiotics. The possibility of multiple factors also cannot be ruled out.
Factors producing low urea were referred to by mentioning that the patient had no liver disease, low protein diet or malnutrition, but the factor leading to disproportionately high creatinine in relation the urea was not alluded to: i.e. excessive muscle damage produced by the abscess.
Comment Reviewer Jesús Garrido MD. Unidade de Nefrologia e Diálise. Hospital São Teotónio de Viseu. Viseu. Portugal
Musso et al. describe an interesting case report, in which drug nephrotoxicity, especially by amikacin, course with an acute renal failure with normal plasmatic urea levels.
Aminoglycosides are prototype drugs having nephrotoxicity as major side effect. Number of patients developing nephrotoxicity increases with duration of therapy reaching 50% with 14 days or more of treatment. Classically it presents as acute tubular necrosis which is generally non-oliguric. Other features include: proximal tubular dysfuntion, enzymuria, proteinuria, glycosuria, hypokalemia, hypomagnesemia... Recovery is slow and requires 4-6 weeks.
Despite its nephrotoxic potential, the aminoglycosides antibiotics are still considered an important agent against life-threatening infections. The goal of identifying and reducing this toxicity has attracted much effort during the last years. A precise monitoring of proximal tubule function to discover the early renal injury could avoid worst consequences. A fast marker of tubular dysfunction in this pathology is the urinary N-acetyl-B-D-glucosaminidase (NAG) activity, that increase in case of proximal tubular damage. Several nephroxicity-ameliorating agents have been described with more or less efficiency as: beta-blockers, Vitamins, antioxidants (a garlic derived compound S-allylmercaptocysteine, trans-resveratrol...), antiplatelet drugs (trapidil), melatonin, ...
The nephrotoxicity of these drugs is frequent but this case is unusual. The interest of this case is the possibility of getting one more proximal tubular damage marker to identify as soon as possible the renal toxicity.
Received: June 18, 2004.
Received revised July 28, 2004
Published: July 31, 2004
