Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
THE INFLUENCE OF MYCOPLASMA INFECTION ON ACUTE MYOCARDIAL INFARCTION
Igor P. Arleevskiy1, Olga A. Chernova2, Indus N. Saphin1,
Maxim V. Trushin2, Vladislav M. Chernov2
1Kazan Medical Academy, 2Kazan Institute of Biochemistry and Biophysics
Kazan, Russia
mtrushin @ mail.ru
Rev Electron Biomed / Electron J Biomed 2006;3:5-10.
Comment of the reviewer Prof. José María Eiros Bouza MD. PhD. Titular de Microbiología de la Facultad de Medicina de la Universidad de Valladolid.
Comment of the reviewer Dr. Ramón Díaz-Alersi MD. Medicina Intensiva. Hospital Puerto Real. Cádiz. España.
We studied haemostatic, immunologic and microelement parameters in patients with acute myocardial infarction, some infected with mycoplasma and some not. Clinical patterns of acute myocardial infarction in the mycoplasma-infected patients were also investigated. Infection with mycoplasmas and other microorganisms of the TORCH group (Toxoplasma, Others, Rubella, Chlamidia) may be risk factors for the development of acute myocardial infarction. However, mycoplasma-infected patients did not show increased levels of postinfarction complications.
KEY WORDS: acute myocardial infarction, mycoplasma, atheromatous plaque, risk factor
RESUMEN
Hemos estudiado los parámetros hemostáticos, inmunológicos y bioquímicos de pacientes con infarto agudo de miocardio, unos infectados con mycoplasma y otros no infectados. También fueron investigados los parámetros clínicos de pacientes con infarto agudo de miocardio infectados por mycoplasma. La infección por mycoplasma y otros microorganismos del grupo TORCH (Toxoplasma, Otros, Rubella, Chlamidia) pueden ser factores de riesgo para el desarrollo de infarto agudo de miocardio. Sin embargo, los pacientes infectados por mycoplasma no presentan incremento de la tasa de complicaciones postinfarto.
PALABRAS CLAVE: infarto agudo de miocardio, mycoplasma, placa de ateroma, fastor de riesgo
INTRODUCTION
Till now, accumulated data suggest that exacerbation of inflammatory processes in arterial walls, favoring destabilization of atheromatous plaques, can precipitate the development of acute myocardial infarction (AMI). Consequently, the detection of trigger agents for chronic inflammatory reactions appears to be significant1-3. Persistent infections associated with microorganisms of the TORCH group (Toxoplasma, Others, Rubella, Chlamidia) including mycoplasmas have been suggested to participate in these processes4-7.
The intent of our study was to elucidate clinical patterns of AMI in mycoplasma-infected patients, and the possible role of mycoplasma infection in the development of the disease.
MATERIALS AND METHODS
Investigations were carried out in the medical ward of the Republican Clinical Hospital №3 of the Tatarstan Ministry for Public Health, the Laboratory of Molecular Pathogenesis of the Kazan Institute of Biochemistry and Biophysics, and the Republican Center for Family, Maternity and Childhood Welfare of the Tatarstan Ministry for Public Health.
76 patients with AMI (age 25 to 75 years, average age 54) residing in the medical ward of the Republican Clinical Hospital №3 participated in the study. The diagnosis of AMI was confirmed by clinical, instrumental and laboratory data.
There were 57 males (average age – 52.3 yrs) and 19 females (average age – 60.4 yrs) in the study group, of which 54 patients were mycoplasma-infected, and 22 patients not infected. Both groups did not differ in the location of the infarction, age, sex, or therapy applied. There were also 40 healthy patients (age 30 to 50 years, average age – 35 yrs). The difference between average ages in the experimental and control groups was insignificant.
Twelve-lead electrocardiographic investigations were repeatedly made.
In patients with AMI and controls, blood samples were tested for mycoplasma infections and carrier state of antibodies to microorganisms of the TORCH group. We also monitored the hemocoagulation parameters, levels of IgG, IgА, IgМ and circulating immune complexes (CIC), concentrations of some macroelements and microelements (strontium, zinc, copper, iron) as well as frequencies of some AMI complications.
Blood and urine was taken during the first three days of admission. Material from atheromatous plaques of coronary arteries of autopsied patients was tested for the presence of mycoplasmas; levels of strontium and zinc were also measured. All procedures were performed according to the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects [http://ohsr.od.nih.gov/guidelines/helsinki.html].
To detect mycoplasmas, a polymerase chain reaction (PCR) with rDNA oligonucleotide sequences as universal primers for revealing the human-specific mycoplasmas8 as well as transmissive micrography were applied9.The presence of antibodies to microorganisms in blood serum of patients was tested by the enzyme-multiplied immunoassay applying commercial kits produced by SPF "Litech" (Russia) and "Vector-Best" (Russia)10.Contents of macroelements and microelements (iron, copper, zinc, strontium) in clinical material were detected by atomic absorption spectroscopy11. Investigation of the serum IgG, IgA, IgM content was performed using radial immunodiffusion12.
The level of total complement was determined in standard units CH-5013.
CIC were revealed by their sedimentation in polyoxyethylene glycol-600014. Calculation of thrombocytes and thrombocyte aggregation were estimated by the "Biola" laser aggregometer15.
Antithrombin III (AT-III) and activated partial thromboplastin time (aPTT) were detected by the methods of Abilgaard16 and Caen17, respectively.
Transmissible microscopy of atheroma samples was done according to Brown9 with some modifications. Material was fixed with 2.5% glutaric aldehyde on phosphate mixture (pH 7.2), then, treated with 1% solution of osmium oxide during 4 h with addition of 2.5 mM saccharose. After dehydration in ethanol of ascending concentration, 100% acetone and oxidopropylene, material was perfused by epon-812. Thin slices obtained using ultramicrotome LKB-III (Sweden) were contrasted with a saturated water solution of uranyl acetate and then with a solution of zinc citrate.
Statistical analysis of the data was performed according to a manual18. A p value of <0.05 was considered significant. Data are presented as mean ± standard deviation.
RESULTS
Mycoplasma infections were detected in 52% of patients: all patients with AMI had also antibodies to TORCH microorganisms. In the control group, 5% of patients were infected with mycoplasmas: antibodies to cytomegalic virus and rubella were also detected (12.5% и 2.5%, respectively).
In the mycoplasma-infected patients, antibodies to cytomegalic virus, toxoplasma and rubella were detected more often than in non-infected patients. Antibodies to chlamidia were more rare (38.5 and 27.3%; 26.9* and 13.6%; 15.4* and 9.3%, 3.8 and 4.6%, respectively; р<0.05). Pathologies in the urogenital system were more frequent (31.8%) in the mycoplasma-infected group of AMI patients in comparison with the non-infected subunit (15.0%).
Increased levels of IgG, IgM and circulating immune complexes as well as decreased levels of total complement were found in the serum of mycoplasma-infected patients (Table 1).
In the mycoplasma-infected patients, thrombophilia was revealed by the decreased activated partial thromboplastin time, the increased spontaneous and induced platelet aggregability, and the tendency to increased level of fibrinogen (Table 1). These facts could not be explained by the anticoagulant therapy, since it was equal in all groups of patients.
As a result of ultracytostructural investigation of coronary artery atheromas in the mycoplasma-infected patients, bodies from 0.2-0.6 µm and their fragments belonging to vegetative cells as well as nannoforms of mycoplasmas19 were detected among villi at outer membranes of endotheliocytes and in cytoplasm of macrophages localized in the subendothelial stratum. In this case, statistically significant increases in strontium and zinc were detected in atheromas of the mycoplasma-infected patients (12.28 ± 2.53 µg/mL*; 44.6 ± 2.02 µg/mL**; *p<0.05, **p<0.01, respectively) in comparison with the non-infected ones (2.49 ± 0.29 µg/mL; 24.7 ± 2.71 µg/mL, respectively). In blood serum of the mycoplasma-infected patients, strontium levels were increased while zinc levels were decreased (Table 1).
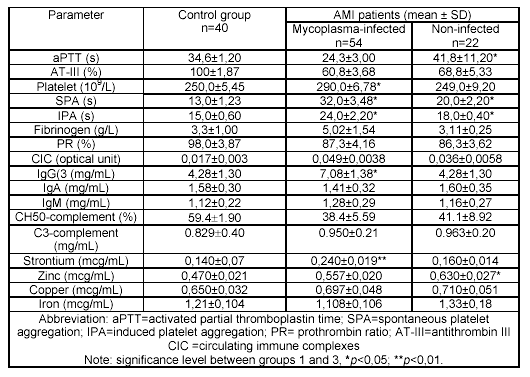
Table 1. Haemostatic, immunologic parameters and content of macroelements
and microelements in the blood serum of people under study.
As a result of this investigation into the frequency of postinfarction complications in mycoplasma-infected and non-infected patients, a tendency toward mild disease was detected: the low rate of ventricular tachycardia, ventricular fibrillation, atrioventricular block, premature postinfarction stenocardia, as well as the low hospital death rate support this conclusion (Fig 1).
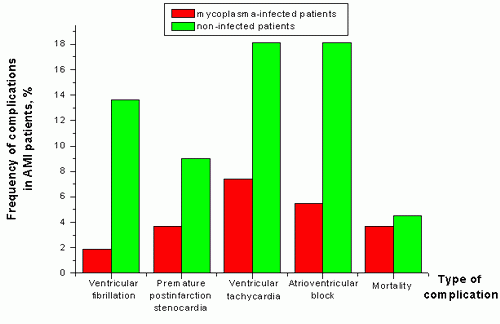
Figure 1. Frequency of complications in AMI patients (in %).
DISCUSSION
Many authors have reported the prevalence of persistent mycoplasma infections in humans. These microorganisms possess unique adaptive abilities enabling them to overcome the various defenses of eukaryotes and higher organisms20. In humans, mycoplasmas cause proliferation of infectious agents; abnormalities in microbiocenosises; respiratory, urogenital, neurodegenerative diseases; and disorders of immunity and homeostasis 20,21. It was shown in the experimental studies that chronic mycoplasma infections are usually accompanied by increasing vascular wall permeability, microcirculation disturbance and thrombosis22. The possible role of mycoplasma infections in the development of AMI was shown6,7.
The limited biochemical competence of these microorganisms make them dependent on higher organisms. Mycoplasmas may reside outside or inside the host cell but are always closely attached to cell membranes. Consequently, mycoplasmas are called "membrane parasites". Mycoplasmas are bacteria without cell walls. To synthesize their own membranes, mycoplasmas need cholesterol, which they extract from host cell membranes. This "membrane parasitism" may determine the characteristics of the pathogenesis in humans with persistent mycoplasma infection19.
Persistent mycoplasmas may be trigger factors in coronary disease, destabilization of atheroma and development of AMI. Biochemical mechanisms of the effects may be connected with reactivity of nonspecific signal systems typical for the persistent infections 23,24 . Mycoplasmas are called "elite" parasites for their ability to modulate the reactivity of specific and nonspecific signal pathways of the host organism (including the synthesis of nitric oxide that probably suppress pathological processes connected with activation of SOD (superoxide dismutase)-producing and NO-synthesizing systems25,26. This is probably an explanation of the tendency to mild AMI we observed in our mycoplasma-infected patients.
Molecular origins of the biochemical, immunological and clinical peculiarities revealed in the mycoplasma-infected AMI patients ought to be investigated. However, the results of our studies allow us to suggest that infections mycoplasmas (and other infectious agents of the TORCH group) may be risk factors in the development of AMI. Mycoplasmas may transfer from person to person and from mother to child27. Taking into account the distribution of these infections, an additional aspects regarding prophylaxis of cardiovascular diseases should be considered.
REFERENCES
- 1. Moreno PR, Berhardi VH, Lopez-Cucllar Jl. Macrophages. Smooth muscle cells and tissue factor in unstable angina: implications for cell mediated trombogenecity in acute coronary syndromes. Circulation 1996; 94: 3090-3097.
2. Ridker PM, Rifai N, Pfefer MA. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events (CARE) investigators. Circulation 1998; 98: 839-844.
3. Yasunobu Y, Hayashik K, Shingu T, Yamagota T, Kajiyama G, Kambe M. Coronary atherosclerosis and oxidative stress as reflected by autoantibodies against oxidized low-density lipoprotein and oxysterols. Atherosclerosis 2001; 155: 445-453.
4. Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis. Circulation 1997; 96: 409-410.
5. Muhlestein J. Chronic infection and coronary artery disease. Med Clin N Amer 2000; 84: 123-148.
6. Higuchi ML, Ranures JAF. Infections agents in coronary atheromas: a possible role in the pathogenesis of plaque rupture and acute myocardial infarction. Rev Inst Med Trop S Paulo 2002; 44: 217-224.
7. Higuchi ML, Reis MM, Sambiase NV, Palomino SA, Castelli JB, Gutierrez PS, Aiello VD, Ramires JA. Coinfection with Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured plaques associated with acute myocardial infarction. Arq Bras Cardiol 2003; 81: 12-22.
8. Wang H, Kong F, Jelfs P, James G, Gilbert GL. Simultaneous detection and identification of common cell culture contaminant and pathogenic Mollicutes strains by Reverse line blot hybridization. Appl Environ Microbiol 2004; 70: 1483–1486.
9. Brown S, Teplitz M, Revel JP. Interaction of mycoplasmas with cell cultures, as visualized by electron microscopy. Proc Natl Acad Sci USA 1974; 71: 464-468.
10. Frimmel G, ed. Immunological methods of investigation. Moscow: Medicine, 1987. In Russian. 11. Varma A, ed. Handbook of Atomic Absorption Analysis. Vol. I. Boca Raton: CRC Press, 1985.
12. Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigen by single radial immunodiffusion. Immunochem 1965; 2: 235-240.
13. Morenz J. The technic of complement fixation reaction. Z Med Labortech 1970; 11: 249-271.
14. Lambert PH, Creighton WD, Miescher PA. Detection of antibodies and soluble antigen-antibody complexes by precipitation with polyethylene glycol. J Immunol 1973; 111: 1219-1227.
15. Gabbasov ZA, Popov EG, Gavrilov IYu, Posin Eya, Markosyan RA. A new methodical approach to research of platelet aggregation in vitro. BEBM 1989; 2: 437-439.
16. Abilgaard U, Lindahl AK, Lacobson PB, Sandset PM. Tissue factor pathway inhibitor with high anticoagulant activity is increased in post-heparin plasma and in plasma from cancer patients. Blood Coagul Fibrinolysis 1991; 2: 713-721.
17. Caen JP, Sultan Y, Larrieu MJ. A new familial platelet disease. Lancet 1968; 2: 203-204.
18. Sokal RR, Rohlf FJ. Biometry. The Principles and Practice of Statistics in Biological Research. San Francisco: Freeman, 1969.
19. Chernov VM, Mukhametshina NE, Gogolev YuV, Abdrakhimov FA, Chernova OA. Adaptive reactions of mycoplasmas in vitro: "viable but unculturable forms" and nanocells of Acholeplasma laidlawii. Microbiology (Moscow) 2005; 74: 428-433.
20. Razin Sh, Herrmann R, ed. Molecular biology and pathogenecity of mycoplasmas. New York: Plenum Publishers, 2002.
21. Borkhsenius SN, Chernova OA, Chernov VM, Vonskii M, ed. Mycoplasmas. St. Petersburg: Nauka, 2002. In Russian.
22. Chernova OA, Mal'tseva LI, Chernov VM, Popova NV. Persistence of mycoplasma in humans may be an indicator of pathology of microbiocenosis. Dokl Akad Nauk 1999; 366: 285-288.
23. Belova LA. Biochemistry of inflammatory processes and vessel damage. The role of neutrophils. Biochemistry (Moscow) 1997; 62: 659-668.
24. Pokrovskaya EV. Atherosclerosis and immune system. Kardiologia 2001; 41: 69-79. In Russian.
25. Mason RP. Nitric oxide mechanisms in the pathogenesis of global risk. J Clin Hypertens (Greenwich) 2006; 8: 31-38.
26. Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 1998; 62: 1094-1156.
27. Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev 2005; 18: 757-789.
The co-infection by Mycoplasma pneumoniae may represent an important co-factor for plaque instability, leading to coronary plaque thrombosis and acute myocardial infarction, since larger amount of these bacteria strongly correlated with histological signs of more vulnerability of the plaque. The present paper entitled "The influence of mycoplasma infection on acute myocardial infarction" conducted by Arleevskiy et al involved 76 patients with AMI (age of 25 to 75 years, average age of 54) and the diagnosis was confirmed according to the clinical, instrumental and laboratory data. The experimental group was divided into two subunits: the mycoplasma-infected patients and non-infected ones. Both subunits did not differ in the AMI localization, age, sex and applied therapy.
In summary It was shown that infection with mycoplasmas together with other microorganisms mght be a risk factor for the development of acute myocardial infarction.
Es bien conocida actualmente la relación entre la inflamación y la enfermedad arteriosclerótica y se ha hipotetizado que diversas infecciones crónicas, en especial por C. pneumoniae y M. pneumoniae pueden contribuir al mantenimiento de un estado inflamatorio que favorezca la progresión de la enfermedad y que se asocie con una mayor probabilidad de sufrir IAM e incluso, que estos sean más graves o de peor evolución. Los estudios clínicos publicados demuestran una gran prevalencia de esas infecciones en los pacientes con IAM, sin embargo, ninguno hasta ahora ha conseguido modificar el pronóstico o la evolución mediante el empleo de antibióticos específicos contra estos microorganismos. Esto último hace que no pueda descartarse que se trate de una relación inocente.
Este artículo muestra las diferencias entre una población sana y otra con IAM en cuanto a marcadores de infección por micoplasma, así como la evolución de los pacientes con IAM.
Received, June 13, 2006. Received reviewed November 7, 2006
Comment of the reviewer Prof. José María Eiros Bouza PhD. Titular de Microbiología de la Facultad de Medicina de la Universidad de Valladolid.
Comment of the reviewer Dr. Ramón Díaz-Alersi MD. Medicina Intensiva. Hospital Puerto Real. Cádiz. España.
Correspondence: Dr. Maxim V. Trushin,
Kazan Institute of Biochemistry and Biophysics,
Russian Academy of Sciences, Lobachevskiy str. 2/31, P.O. Box 30,
Kazan 420111, Russia;
mtrushin @ mail.ru; Tel.:007-843-2319026.
Published, November 15, 2006
