Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
EFFECTS OF ZINGIBER OFFICINALE ON LIVER FUNCTION OF MERCURIC CHLORIDE-INDUCED HEPATOTOXICITY IN ADULT WISTAR RATS
1Ezeuko Vitalis C.*, 3Nwokocha Chukwuemeka R.,
3Mounmbegna Philippe E. 3Nriagu Chinonso C.
Department of Anatomy1 and Biochemistry3, Madonna University, Elele Campus, Rivers State.
Departmant of Physiology2, Delta State University, Abraka, Delta State.
Nigeria
Rev Electron Biomed / Electron J Biomed 2007;3:40-45
Comment of the reviewer Maxim V Trushin PhD. Laboratory of Pathogenesis. Kazan Institute of Biochemistry and Biophysics. Russian Academy of Sciences. Kazan Russia
Comment of the reviewer Erhan Süleymanoglu PhD. G.U.E.F., Department of Pharmaceutical Chemistry, Gazi University. Gazi Mahallesi, Ankara. Turkey
ABSTRACT:
This research is aimed at investigating the hepatotoxic effect of mercury chloride and effects of zingiber officinale on this hepatotoxicity. These were carried out via estimation of liver function tests. Fifteen adult wistar rats were used for the experimental investigations. They were grouped into three: groups 1, 2 and 3 respectively. Animals in group 1 served as the control group. Group 2 consists of rats administered with mercuric chloride (5mg/kg body weight) through intraperitoneal injection. Group three consists of rats administered with mercuric chloride (5mg/ kg body weight) through intraperitoneal injection and fed with diet supplemented with ginger. The experimental period lasted for twenty days. The rats were sacrificed on the twentieth day after being starved for twelve hours. The blood samples collected by cardiac puncture and placed in appropriately labeled bottles for the various assays.
The data obtained was analyzed using the students' t-test distribution. Means of the data was obtained and recorded as mean + standard deviation. There was an increase in the weight of the rats in the control group. There was a decrease in the mean weight of the rats treated with mercury only while in the rats treated with mercury and ginger, there was an increase in the mean weight. The bilirubin level of the rats treated with mercury is significantly higher (P<0.05) than the rats in the control group while there was no statistically significant difference (P>0.05) between the rats in the control group and the rats treated with mercury and ginger. The aspartate aminotransaminase level, alanine aminotransferase level and alkaline phosphatase level are significantly lower (P<0.05) in the rats of the control group than both the rats treated with mercury only and the ones treated with mercury and ginger. These were lower in the rats treated with ginger than the rats treated with mercuric chloride alone. These reduction is however not statistically significant and it presents that these could be dosage related.
These results indicate that mercuric chloride is hepatotoxic and that zingiber officinale has a protective effect on this hepatotoxicity.
Key words: Bilirubin, aspartate aminotransaminase, alanine aminotransferase, alkaline phosphatase.
RESUMEN:
Esta investigación tiene por objeto la investigación de los efectos hepatotóxicos de cloruro de mercurio y los efectos de la zingiber officinale sobre esta hepatotoxicidad. Éstas, se llevaron a cabo a través de la estimación de las pruebas de función hepática. Quince ratas wistar adultas fueron utilizados para la investigacion experimental. Las ratas se divieron en tres grupos: grupos 1, 2 y 3, respectivamente. Los Animales en el grupo 1 sirvieron de grupo de control. Grupo 2 consta de ratas a las que se administró cloruro de mercurio (5mg/kg peso corporal) mediante inyección intraperitoneal. El grupo consta de tres ratas a las que se administró cloruro de mercurio (5 mg / kg de peso corporal) mediante inyección intraperitoneal y fueron alimentadas con la dieta suplementada con jengibre. El período experimental duró veinte días. Las ratas fueron sacrificados a los veinte días después de un periodo de ayuno de doce horas. Las muestras de sangre fueron recogidas por punción cardiaca y se colocaron en frascos debidamente etiquetados para los diferentes ensayos.
Los datos obtenidos se analizaron mediante la prueba t de student. Se obtuvo la media y la desviación estándar. Hubo un aumento en el peso de las ratas en el grupo control. Hubo una disminución en el peso medio de las ratas tratadas con mercurio sólo mientras que en las ratas tratadas con el mercurio y el jengibre, hubo un aumento en el peso medio. El nivel de bilirrubina de las ratas tratadas con mercurio es significativamente mayor (P < 0.05) que las ratas en el grupo control, mientras que no hubo diferencias estadísticamente significativas (P > 0,05) entre las ratas en el grupo control y las ratas tratadas con mercurio y Ginger. El nivel de aspartato aminotransaminasa, la alanina aminotransferasa y el nivel de fosfatasa alcalina fueron significativamente más bajos (P < 0.05) en las ratas del grupo de control que en las ratas tratadas con mercurio y sólo los tratados con el mercurio y el jengibre. Estos fueron menores en las ratas tratadas con jengibre que en las ratas tratadas con cloruro de mercurio solo. Esta reducción sin embargo, no fue estadísticamente significativa y se podría relacionar con la dosis.
Estos resultados indican que el cloruro de mercurio es hepatotóxico y que zingiber officinale tiene un efecto protector sobre su hepatotoxicidad.
Palabras clave: Bilirrubina, aspartato aminotransaminase, alanina aminotransferasa, fosfatasa alcalina.
INTRODUCTION
Mercury intoxication has been a public health problem for many decades1. Consideration of the role of environmental factors in determining susceptibility to mercury has recently been renewed by evidence from epidemiologic studies in the Amazon2, the Republic of the Seychelles3 and the Faroe Islands4. Although many of these populations have been exposed to similar doses of mercury through the consumption of fish and seafood, some populations have experienced subsequent neurotoxic effects, whereas others have not5. Since the epidemic mercury poisoning from contaminated fish consumption in Minamata, Japan in the late 50s6, 7, mercury has been one of the most dramatic and best documented examples of bio-accumulation of toxins in the environment, particularly in the aquatic food chain8.
The body accumulates ingested amounts of mercury in the kidney, brain, liver and other tissue including the hair9. Elemental mercury and its metabolites have the toxic effect of denaturing biological protein, inhibiting enzyme and interrupting membrane transport and the uptake and the release of neurotransmitters. Rarely, significant exposure to mercury can cause acrodynia or "pink disease", involving a pink rash on the extremities, paresthesias and pain10.
Mercury is absorbed throughout the intestine and absorption is possible through most biologic membranes11-12 such as albumin and other sulfur-containing proteins13-14. Mercury recycles through the enterohepatic system in adults12, 15 and is excreted primarily in the feces16. Various works had been carried out on the effects of nutrients on transport, distribution and retention of mercury and on the overall effects of mercury on the metabolism of protein, carbohydrates, lipids and other metabolites17-20.
zingiber officinale, commonly called ginger, is a large, fleshy rhizome which in fresh state has a characteristic staghorn-like appearance. The leaves are occasionally used for flavouring21. The main constituents of ginger include volatile oil (bisabolene, cineol, phellandrene, citral, borned, citronellol, geranial linalool, limonene, zingeberol, zingeberene, camphene), oleoresin (gingerol, shogoal), phenol (gingeol and zingerone), proteolytic enzymes (zingibain), vitamin B6, vitamin C, calcium, magnesium, phosphorus, potassium, linoleoc acid22. The pungency and aroma of ginger are because of the gingerol and volatile oil respectively21.
Ginger is economically valuable as spices and perfumes. It is consumed in many parts of the world either as a bulk (raw or cooked) or added to meal. However, cooked ginger will have an increased pungency but decreased freshness23. Ginger tea, prepared by cooking slices of fresh ginger for a few minutes is a spicy and healthy drink enjoyed in hot tropic climates (Indonesia) and in the chill Himalayas24.
The root is used to stimulate blood flow to the extremities in cases of blood circulation problems, chilblains or muscle cramps. For fever, it is used to promote perspiration. It increases gastric secretions and is useful in slow or difficult digestion, fratulence and colic. A gargle made with an infusion alleviates sore throat. Ginger is also useful in alleviating menstrual cramps. It is useful in colds, flu and other infectious diseases and also in alleviation of nausea, vomiting of motion sickness25
Many researchers have different opinions as regards with the adverse effects of ginger. Food and drug agency classify it as generally recognized as safe. However in large doses, ginger may cause gastrointestinal distress in some individuals, particularly if ingested on an empty stomach. Large doses also have the potential of causing CNS depression and cardiac disturbances.
Liver function tests serve as diagnostic aids when a metabolic process has been disturbed. These include bilirubin test, alkaline phosphatase test, aspartate aminotransferase test and alanine aminotransferase test. for bilirubin estimation, the method described by Jendrassik et al.26 and Sherlock27 was used, for alanine transaminase (ALT) estimation the method described by Reitman et al.28 and Schmidt et al.29 was used, for aspartate transaminase (AST) estimation, the method described by Reitman et al.28 used and for alkaline phosphatase (ALP) estimation the method described by Rec. GSCC (DGKC)30 was used.
This research is aimed at investigating the hepatotoxic effect of mercury chloride and effects of zingiber officinale on this hepatotoxicity. These were carried out via estimation of liver function tests.
MATERIALS AND METHOD
zingiber officinale: this was bought from Adazi Ani market, dried and then chopped and mixed with the rat chow in a ratio of 1:9 by weight.
Experimental animals: fifteen adult wistar rats whose weights ranged between 168 and 262g before the experiment and between 127 and 268 at the end of the experiment, obtained from the animal house of Madonna University, Elele campus, Rivers State, Nigeria were used for the experimental investigations. The animals were allowed to acclimatize for two weeks fed with normal rat chow and tap water ad libitum before the experiment took off. They were grouped into three in a ratio of five rats to a cage and labeled group 1, 2 and 3 respectively. Animals in group 1 served as control, administered normal saline according to the body weight and normal rat chow throughout the period of the experiment. Group 2 consists of rats administered with mercuric chloride (5mg/kg body weight) through intraperitoneal injection and fed with normal rat chow. Group three consists of rats administered with mercuric chloride (5mg/ kg body weight) through intraperitoneal injection and fed with diet supplemented with 10% ginger throughout the experimental period. The experimental period lasted for twenty days.
Collection of blood samples: the rats were sacrificed on the twentieth day after being starved for twelve hours. The rats put in chloroform and the blood samples collected by cardiac puncture and placed in appropriately labeled bottles for the various assays for bilirubin test, alkaline phosphatase test, aspartate aminotransferase test and alanine aminotransferase test.
Statistical analysis: the data obtained was analyzed using the students' t-test distribution. Means of the data was obtained and recorded as mean ± standard deviation. Values were statistically significant when probability is less than 0.05 (P < 0.05).
RESULTS
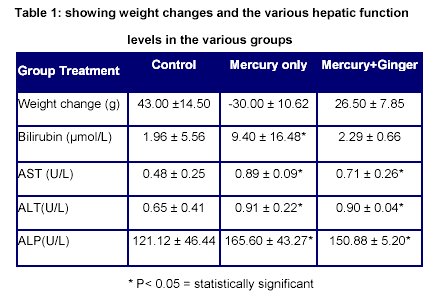
From the table above, there was an increase in the weight of the rats in the control group. There was a decrease in the mean weight of the rats treated with mercury only while in the rats treated with mercury and ginger, there was an increase in the mean weight.
The bilirubin level of the rats treated with mercury is significantly higher (P<0.05) than the rats in the control group while there was no statistically significant difference (P>0.05) between the rats in the control group and the rats treated with mercury and ginger.
The aspartate aminotransaminase (AST) level in the rats of the control group is significantly lower (P<0.05) in the rats of the control group than both the rats treated with mercury only and the ones treated with mercury and ginger. However AST level of the rats treated with mercury and ginger is lower than those treated with mercury only.
The alanine aminotransferase (ALT) level in the rats of the control group is significantly lower (P<0.05) in the rats of the control group than both the rats treated with mercury only and the ones treated with mercury and ginger. However ALT level of the rats treated with mercury and ginger is lower than those treated with mercury only.
The alkaline phosphatase (ALP) level in the rats of the control group is significantly lower (P<0.05) in the rats of the control group than both the rats treated with mercury only and the ones treated with mercury and ginger. However ALP level of the rats treated with mercury and ginger is lower than those treated with mercury only.
DISCUSSION
The loss in the mean weight of the rats treated with mercury shows that toxicity must have been induced in the rats which led to loss appetite, decrease in food consumption and weight loss. This conforms to the previous report (31)
The higher bilirubin level in the rats treated with mercuric chloride shows that mercuric chloride is highly toxic to the liver functions. This increase in bilirubin concentration in the serum indicate that bile is not being excreted and/or that too much hemoglobin is being destroyed and/or that the liver is not actively treating the hemoglobin it is receiving (32) and could therefore lead to jaundice. This is, however, subject to further research. The reduction in bilirubin level observed in rats treated with mercuric chloride and supplemented with ginger indicates that ginger has a protective action on mercuric chloride induced hepatotoxicity in general and on the high bilirubin level induced by the toxicity in particular.
The raised aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels in the rats treated with mercuric chloride further indicate mercuric chloride-induced hepatoxicity. There are observed reductions in the rats supplemented with ginger. The effects of ginger in alleviating this condition could be related to dosage and/or long term administration. This is, however, subject to further study.
REFERENCES
-
1. World Health Organization. Methyl mercury. Environ Health Crit 1990; 101:144
2. Lodenius M. and Malm O. Mercury in the Amazon. Rev Environ Contam Toxicol 1998; 157:25-52.
3. Myers GJ, Davidson PW, Shamlaye CF, Axtell C, Cernichiari E, Choisy O, Choi A, Cox C, Clarkson TW. Effects of prenatal methyl mercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology 1997; 18:819-830.
4. Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive defeit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997; 19:417-428.
5. Myers GJ and Davidson PW. Prenatal methylmercury exposure and children-neurologic, developmental and behavioral research. Environ Health Perspect 1998; 106(suppl 3):841-847.
6. Tsubaki T and Irukayama K. Minamata Disease: Methylmercury poisoning in Minamata and Niigata, Japan. Amsterdam: Elsevier, 1977; 281.
7. Weiss B. Long ago and far away: a retrospective on the implications of Minamata. Neurotoxicology1996; 17:257-263.
8. Boudou A and Ribeyre F. Mercury in the food web: accumulation and transfer mechanisms. Metal Ions Biol System1997; 34:289-319.
9. Kojima S, Shimada H, Kiyozumi M. Comparative effects of chelating agents on distribution, excretion and renal toxicity of inorganic mercury in rats. Res Commun Chem. Pathol Pharmacol 1989; 64:471-484.
10. Katzung GB. Basic and Clinical Pharmacology. 9th Edition. McGraw Hill Company, New York.2004; 970-981.
11. Clarkson TW. The pharmacology of mercury compounds. Ann Rev Pharmacol 1972; 12:375-406.
12. Norseth T and Clarkson TW. Intestinal transport of 203Hg-labelled methyl mercury chloride. Role of biotransformation in rats. Arch Environ Health 1971; 22:568-577.
13. Yasutake A, Hirayama K, Inoue M. Interaction of methyl mercury compounds with albumin. Arch Toxicol 1990; 64:639-643.
14. Cambar J, Boudou A, Hocquellet P, Faugere JG. Mercury fixation in different subfractions of human serum albumin separated by polyacrilamide gel electrophoresis. Eur J Toxicol Environ Hyg 1975; 8:201-204.
15. Norseth T. Biliary excretion and intestinal reabsorption of mercury in rats after injection of methyl mercuric chloride. Acta Pharmacol Toxicol 1973; 33:280-288.
16. Rowland IR, Robinson RD, Doherty RA. Effects of diets on mercury metabolism and excretion in mice given methylmercury: role of gut flora. Arch Environ Health 1984; 39:401-408.
17. Menon NK and Lopez RR. The effects of mild congenital methylmercury intoxication on the metabolism of 3-hydroxybutyrate and glucose in the brains of suckling rats. Neurotoxicology 1985; 6:55-61.
18. Gill TS, Tewari H, Pande J. Use of the fish enzyme system in monitoring water quality:effects of mercury on tissue enzymes. Comp Biochem Physiol C 1990; 97:287-292.
19. Kutznetsov DA. Paradoxical effects of methyl mercury on mitochondrial protein synthesis in mouse brain tissue. Neurochem Res 1987; 12:751-753.
20. Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) and methyl mercuric chloride (MeHgCl)-induced amino acid release neonatal rat primary astrocytes cultures. Brain Res 1994; 664:133-140.
21. Kikuzaki H, Kawasaki Y, Nakatani N. Structure of antioxidativa compounds of ginger. ACS symposium series 1994; 547:237-243.
22. Kikuzaki H and Nakatani N. Antioxidant effects of some ginger constituents. Journal of food science 1993; 58 (6): 1407-1410.
23. Safra E.J. and Yeshua I. The new encyclopedia Britannica; 15th edition, Encyclopedia Inc 2003; 5:271.
24. Lee IK and Ahn SY. The antioxidant activity of gingerol. Korean Journal of food science and technology 1985; 17 (2): 55-59.
25. Gomez R.A. Amazing power of healing plant. 2nd edition. Inter-American Division Publishing Association USA 2003; 234-235.
26. Jendrassik L, Grof P. Vereinfachte, Photometrische Methoden zur Bestimmung des Blutbilirubins. Biochem Z. 1938; 297: 81-89.
27. Sherlock S. Liver disease. Churchill, London 1951; 204.
28. Reitman S, Frankel S. Calorimetric method for glutamic- pyruvic transaminase and glutamic-oxaloacetic transaminase. Am J Clin Path 1957; 28: 56-70.
29. Schmidt E, Schmidt FW. Determination of serum GOT and GPT. Enzym Biol Clin. 1963;3:1-3.
30. Rec. Gscc (DGKC). Optimised standard colorimetric methods. Journal of Clinical Chemistry and Clinical Biochemistry. 1972; 10:182.
31. American Petroleum Institute. The toxicity of lead. Medical report No. EA7102 Washington D.C. 1981.
32. Devlin T.M. Textbook of biochemistry with clinical correlations. 5th edition. Wiley-Liss Publishers, New York 2002.
Received November 1, 2007. Received reviewed November 30
Published December 31, 2007
