Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
DEPOSITION STUDIES USING
MULTIPURPOSE SOLUTION ON
HYDROPHILIC CONTACT LENSES
A. Arora1, A. Ali2, R. K. Khar2, M.T. Zzaman1.
1Kiet School of Pharmacy. Ghaziabad-Meerut Road. Ghaziabad
2Department of Pharmaceutics. Faculty of Pharmacy. Jamia Hamdard, Hamdard Nagar.
New Delhi. India
alkamailin @ yahoo.co.in
Rev Electron Biomed / Electron J Biomed 2009;1:10-17
Comment of the reviewer Prof. Pilar Muñiz Rodriguez. PhD. Professor of Biochemistry and Molecular Biology, Faculty of Science. University of Burgos. España
Comment of the reviewer Victoria Valls Bellver PhD. Biochemistry. Department of Pediatry and Ginecology. University of Valencia. España
SUMMARY
In the tears lysozyme and albumin are also present besides other constituents. All these constituents form a biofilm on the hydrophilic contact lenses - minutes after the lens is placed in the eye. These deposits if not removed make the contact lens translucent and impair visual acuity. For the removal of deposit multipurpose solution is used.
In the study, deposits of lysozyme and albumin were made on hydrophilic contact lenses deliberately. These deposits laden contact lenses were then treated with multipurpose solution for 12 hrs. The extent of removal of these deposits by the action of sodium citrate present in multipurpose solution was assessed by measuring albumin and lysozyme quantitatively by using standard analytical procedures.
It was observed that 0.1% of sodium citrate could remove lysozyme and albumin efficiently. Albumin deposited more as compared to lysozyme and non ionic hydrophilic contact lenses are less prone to deposition than ionic. Any further increase in sodium citrate was not desirable.
KEY WORDS: Lysozyme. Albumin. Sodium citrate. Standard tear fluid (STF). Non enzymatic cleaner
RESUMEN
En las lágrimas están presentes lisozima y albúmina, además de otros constituyentes. Todos estos componentes forman una biopelícula en las lentes de contacto hidrofílicas y minutos después de la lente se coloca en el ojo. Estos depósitos, si no se eliminan, hacen el contacto con la lente translúcida y afectan la agudeza visual. Para eliminar los depósitos se utiliza una solución multiuso.
En el estudio, se hicieron deliberadamente depósitos de lisozima y albúmina en lentes de contacto hidrofílicas. Estos depósitos sobre los lentes de contacto fueron tratados con la solución multiuso durante 12 horas. El grado de eliminación de estos depósitos por la acción del citrato de sodio en solución multiuso se evaluó mediante la medición de la albúmina y la lisozima cuantitativamente, mediante procedimientos analíticos.
Se observó que el citrato de sodio al 0,1% podría eliminar la lisozima y albúmina de manera eficiente. La Albúmina se depositó más en comparación con la lisozima y las lentes de contacto hidrofílicas no iónicas son menos proclives al depósito que las iónicas. Cualquier aumento posterior de citrato de sodio es indeseable.
PALABRAS CLAVE: Lisocima. Albúmina. Citrato sódico. Fluído standard de lágrimas (STF). Limpiador no enzimático.
INTRODUCTION
In the eye besides tear, other constituents are also present like proteins, lysozyme, albumin and salts including calcium. All these form a lipoprotein surface film on the hydrophilic contact lenses and other contaminants are adsorbed on this film further. The contaminants may be the environmental pollutants such as nicotine, cosmetic ingredients, finger dirt, chemical vapors, water impurities and preservatives/active ingredient from ophthalmic products1.
Certain other lipid secretions from the eye glands (meibomian glands) can also bind to the lens surface, forming a lipoprotein film that is very difficult to remove. All such deposits if not removed then may cause discomfort and impair visual acuity. Microorganisms may further build up on these deposits and the situation further worsens. To remove such deposits, the lenses are to be treated every day with multipurpose solution (MPS) containing a deproteiniser. The deposits not only cause discomfort but also increase the risk of infection causing giant papillary conjunctivitis (GPC)2.
The enzyme cleaners provide effective cleaning but leave around 25% of the lens surface area still coated. One of the functions of multipurpose solution (MPS) is to remove lens deposits when lenses are soaked in the solution overnight. In this way, it extends the useful life of the lens and keeps the lens free from deposits and thereby provides clear vision, comfort and maintain normal eye health3.
During day time the lenses which are previously rinsed with MPS before being worn and during night, the lenses when not in use are soaked in MPS for 7-8 hours. Lens can be worn continuously for 7-8 hours in a day, after this again rinsed and soaked in MPS for 7-8 hours before being worn again4.
MATERIAL AND METHODS
1. Materials
Polyhexanide hydrochloride (PHMB.HCl) was procured from Avecia Biocides, Manchester, U.K. Sodium citrate was obtained from Merck, Mumbai, India and Lysozyme from SRL, Mumbai, India. Albumin was obtained from E Merck, Mumbai, India. FDA group I (Netrafilcon A) hydrophilic contact lenses were used. All other materials were used as received.
2. Preparation of standard tear fluid (STF) containing deposition constituents
STF of pH 7.4 was prepared (Table I).
Albumin and lysozyme (Table II) were added into isotonic STF of pH 7.4 by shaking the flask until a clear solution was obtained. The volume was made and pH was adjusted up to 7.4 using pHmeter.
3. Selection of hydrophilic contact lenses and MPS
Group I (Netrafilcon A) hydrophilic contact lenses were used for the study. For one MPS, six contact lenses were used. The total contact lenses were 42.
Container used: Transparent vial of 10.0 ml capacity were used for the study.
MPS tested: Seven selected MPS were subjected to deposition studies i.e. MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11
4. Method
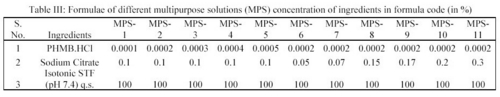
In the deposition studies, two main constituents, which are generally deposited on the surface of the contact lens, are lysozyme and albumin. In the present study the removal of lysozyme and albumin from the deposited hydrophilic contact lenses (CL's) by the action of MPS was studied in order to assess the formulation. Eleven preparations of MPS were prepared and coded (Table III).
All these preparations contain polyhexanide hydrochloride (PMHB.HCl) as the drug and sodium citrate as the deproteiniser and the concentration of the drug varied from 0.0002 to 0.0005% and the concentration of sodium citrate (deproteiniser) varied from 0.05 to 0.30%. In the deposition studies, lysozyme and albumin were added into an isotonic simulated tear fluid (STF) of pH 7.4 in known concentration and the hydrophilic contact lenses were soaked in it for 24 hours at 37°C in order to make coatings of lysozyme and albumin on them i.e. deposits were made on the lenses deliberately and according to the composition as given in tables (Table II and Table III). These lenses were then soaked in MPS for 12 hours and the lysozyme and albumin were estimated in order to assess the deposit removing capacity of MPS 5.
In 42 vials, the artificial tear fluid containing lysozyme and albumin were added (5.0 ml in each vial). In each of 42 vials the hydrophilic contact lens was placed. All the vials were stopper with their respective caps and placed in biological shaker at 37°C. These were shaken for 24 hours. After 24 hours, the lenses were removed with the help of contact lens lifter and placed in separate vial containing 5.0 ml of MPS.
For one MPS, six vials were used and in each vial one lens was placed. These vials were left for 12 hours at room temperature i.e. 25°C. After this these vials were shaken for 5 minutes and lenses were removed. The treated MPS were analyzed for the deposition of lysozyme and albumin, removed from the lenses, by the following methods.
4.1. Estimation of lysozyme
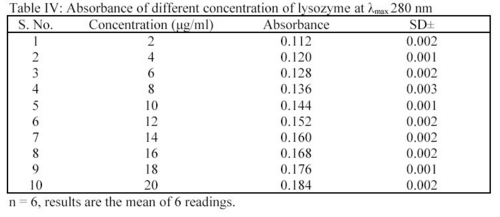
It was determined as per the method of Hu et al7. Different concentrations of lysozyme were prepared in STF of pH 7.4 i.e. 2.0 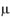 g/ml to 20.0
g/ml to 20.0 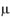 g/ml. The absorbance of the solutions were determined at
g/ml. The absorbance of the solutions were determined at 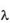 max 280 nm using UV spectrophotometer. From the readings (Table IV), a standard plot was prepared.
max 280 nm using UV spectrophotometer. From the readings (Table IV), a standard plot was prepared.
In deposition studies, the treated MPS preparations were taken and the absorbance were determined at 280 nm. The amount of lysozyme was determined by using the standard plot as per the method. From the readings, a bar chart was plotted for showing the effect of MPS on removal of lysozyme from hydrophilic contact lenses (CL's)6.
4.2. Estimation of albumin
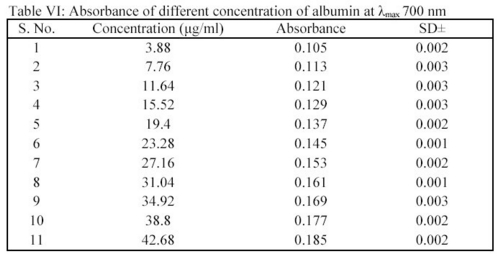
Albumin was determined as per modified Lowry method7. A stock solution of albumin 100 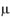 g/ml was prepared in STF of pH 7.4. From this stock solution, an appropriate volume was transferred into a 10.0 ml capacity volumetric flask. To this 1.0 ml of biuret reagent and 1.0 ml of phenol were added. After 5 minutes, volume was adjusted up to 10.0 ml. In this manner, all reaction mixture were prepared containing different concentrations of albumin i.e. 3.88
g/ml was prepared in STF of pH 7.4. From this stock solution, an appropriate volume was transferred into a 10.0 ml capacity volumetric flask. To this 1.0 ml of biuret reagent and 1.0 ml of phenol were added. After 5 minutes, volume was adjusted up to 10.0 ml. In this manner, all reaction mixture were prepared containing different concentrations of albumin i.e. 3.88 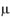 g/ml to 42.68
g/ml to 42.68 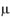 g/ml. Albumin gave intense red color in the presence of biuret agent and phenol. The absorbance of these solutions was measured spectrophotometrically at 700 nm (Table VI).
g/ml. Albumin gave intense red color in the presence of biuret agent and phenol. The absorbance of these solutions was measured spectrophotometrically at 700 nm (Table VI).
From the absorbance and concentration value, a standard plot was drawn. In deposition studies, the treated MPS was taken and to this the biuret agent and phenol were added as the method given above. The absorbance was determined and the amount of albumin was calculated using the standard plot.
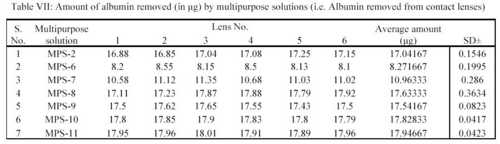
From the readings a bar chart was plotted, showing the effect of MPS solution on removal of albumin (Table VII).
RESULTS:
Seven MPS coded as MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 were tested for their deposits removal capacity and efficiency upon treatment of the deposits laden hydrophilic contact lenses (Table III).
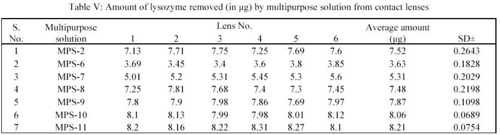
In the study, the deposits of lysozyme and albumin were made on the contact lenses deliberately by soaking them in STF of pH 7.4 containing the above constituents for 24 hours at 37 ± 0.5°C. Hydrophilic contact lenses of group I (Netrafilcon A) were used. The deposits laden contact lenses were then treated with MPS for 12 hours. Sodium citrate (deproteiniser) present in MPS in concentration of 0.1%, 0.05%, 0.07%, 0.15%, 0.17%, 0.20% and 0.30% in MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 respectively removed lysozyme. The average amount of lysozyme in 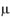 g removed per lens by MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 were 7.52, 3.63, 5.31, 7.48, 7.87, 8.06 and 8.21 respectively (Table V).
g removed per lens by MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 were 7.52, 3.63, 5.31, 7.48, 7.87, 8.06 and 8.21 respectively (Table V).
Similarly average amount of albumin removed per lens in  g by MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 were 17.04, 8.27, 10.96, 17.63, 17.54, 17.83 and 17.95 respectively (Table VII).
g by MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 were 17.04, 8.27, 10.96, 17.63, 17.54, 17.83 and 17.95 respectively (Table VII).
The removal occurred on the surfaces of non-ionic hydrophilic contact lenses, which are FDA approved, group I and group II types8. The albumin was deposited more than lysozyme and the capability of the MPS with sodium citrate used as deproteiniser was more towards lysozyme.
The lysozyme could be removed easily than albumin by the MPS for hydrophilic contact lenses.
0.1% of sodium citrate in MPS for hydrophilic contact lenses is upto the mark. Sodium citrate 0.1% can be used as deproteiniser for removal of lysozyme and albumin present on the surface of hydrophilic contact lenses.
DISCUSSION:
One reason for removing contact lens deposits is to extend the useful life of the contact lens. The more important reasons for cleaning hydrophilic contact lenses are to maintain clear vision, good comfort, and most importantly normal eye health. Undesirable organic substances within the tear film layer, such as lipids, mucoproteins, albumin, immunoglobulin, glycoproteins, mucin and lysozyme combine with inorganic compounds, bacteria and microorganisms to form a complex biofilm deposit on contact lens surface within minutes of placing the lens on the eye. These deposits continue to build on the contact lens surface with successive wearing period, eventually causing discomfort from mechanical irritation of the ocular tissues, as well as blurred vision as the optical quality of the contact lens surface degrades. This biofilm can also act as an antigenic stimulus causing allergic lid reactions such as giant papillary conjunctivitis (GPC).
GPC causes blurred vision, reduced wearing time, redness, itching, stinging, ocular discomfort and mucous discharge. GPC used to be a frequent occurrence with hydrophilic contact lenses but with the advent of multipurpose solution with non enzymatic cleaners like citrate, tris, hydranateTM the incidence of GPC in hydrophilic contact lens wearer has decreased considerably. Sodium citrate is a non enzymatic deproteiniser used in MPS. It's a trivalent anionic molecule with chelating properties. It is effective in removing protein, lipids and polysaccharide deposits from contact lenses surface and breaks calcium bridges which link protein deposits to each other and to the lens. The cleaning activity and no ocular toxicity promote longer lens life. Citrate is therefore used in MPS for the overnight storage of the lens and for rinsing and soaking of the contact lenses. Citrate is a non enzymatic deproteiniser in the MPS.
Tear proteins such as lysozyme and albumin are large multivalent molecules containing both positive and negative local areas of charge. The positively charged sites on protein molecule can form ionic bonds with the negatively charged surface of the ionic contact lens and thereby binding proteins to the surface. One protein layer on to the other therefore builds up within no time, this ionic building is strong type hence to remove such bindings one has to store the lenses overnight and also to clean, rinse the lens by mechanically rubbing with MPS. This cause the removal of deposit coupled by weekly cleaning using an enzymatic cleaner as well.
In the experiment, the non ionic lenses are used because here the binding is not that strong and non enzymatic cleaner which are less allergy prone like sodium citrate has been used. There is no strong binding hence no need of enzymatic cleaner like papain which induce allergy. Hence sodium citrate could solve the purpose and is therefore used as deproteiniser. Binding occurs but not to that extent as in ionic type and this binding is broken by the compound like citrate8.
In order to ascertain the efficiency of MPS preparation towards the removal of deposits from the contact lenses, a deposition study was performed. In the study, lysozyme and albumin were measured quantitatively i.e. removal of these deposits from the surface of the lens has been studied. Seven MPS preparations i.e. MPS-2, MPS-6, MPS-7, MPS-8, MPS-9, MPS-10 and MPS-11 were tested for their deposits removal efficiency upon treatment of the used lenses by them. These preparations differ in the sodium citrate concentration- the deproteiniser. The concentration of sodium citrate varied from 0.05% to 0.3% in these formulations.
In the study, the deposits of lysozyme and albumin were made on the contact lenses deliberately by soaking them in STF of 7.4 containing these components for 24 hours at 37±0.5°C. Group I (Netrafilcon A) were used for the reasons given above. These lenses are non ionic type. These deposits laden contact lenses were then treated with MPS preparation for 12 hours. During the treatment period, the deposits of lysozyme and albumin were removed by the action of sodium citrate present in the MPS. The extent of removal of these deposits was assessed by measuring albumin and lysozyme quantitatively by using standard analytical procedures discussed in the section. It was observed that the preparation containing less amount of sodium citrate i.e. 0.05% in MPS-6 removed less amount of lysozyme and albumin.
It was further ofserved that(the preparation containing more amount of sodium citrate i.e. MPS-7 and MPS-2 (0.07% of sodium citrate MPS-7 and 0.1% in MPS-2) removeÐ these deposits in increasing orders. However, further increase of sodium citrate i.e. beyond 0.1% in the preparation of MPS-8, MPS-9, MPS-10 and MPS-11 remove the deposits, slightly more as sompared to MPS-12ˆbut not significantly. The result of MPS-2 preparation was comparable with other preparations i.e. MPS-8, MPS-9, MPS-10 and MPS-11. This is due to the fact that entire deposits were removed by MPS-2 preparation containing 0.1% of sodium citrate; therefore further increase in the sodium citrate concentration was not desirable i.e. the concentration of sodium citrate in the preparation MPS-8, MPS-9, MPS-10 and MPS-11 were in excess. Hence MPS-2 is an optimized preparation as far as quantity of sodium citrate is concerned.
It was further observed that deposits due to albumin were more as compared to lysozyme. The solution with 0.1% of sodium citrate could remove lysozyme more efficiently than albumin. Non ionic contact lenses - group I (Netrafilcon A) were used because these lenses actually discourage binding within the polymeric network of hydrophilic contact lenses. The binding is weak enough that non enzymatic cleaner like sodium citrate can break it easily.
From the deposition studies it was concluded that MPS-2, the multipurpose solution preparation, gave better results in the removal of deposits from the surface of the hydrophilic contact lens as compared to the other prepa
REFERENCES
- 1. Mandell RB. Contact Lens Practice. 3rd Edition, Charles C. Thomas. Springfield. 1981:44-78.
2.- Stewart-Jones JH, Hopkins GA, Phillips AJ. Drugs and solutions in contact lens practice and related microbiology. In: Phillips AJ, Stone J, eds. Contact Lenses. A Textbook for Practitioner and Student. 2nd ed. London; Boston: Butterworths, 1980, 1-59
3.- Vale J and Cox B. Drugs and the Eye. Contact lens solutions. 2nd Ed. Butterworth, London, 1985.
4.- Dada VK. Textbook of Contact Lenses. Ed.4: Jaypee Brothers, 1996.
5.- Hu J, Tartaglia L, Kohler J, Lett J, Shih K. Gentle Touch, a lens material resistant to protein deposition. CLAO J 1985;21:93-95.
6.- Jung J, Rapp J. The efficacy of hydrophilic contact lens cleaning systems in removing protein deposits. CLAO J 1993;19: 47-49.
7.- Jones L, Evans K, Sariri R, Franklin V, Tighe B. Lipid and protein deposition of N-vinyl pyrrolidone-containing group II and group IV frequent replacement contact lenses. CLAO J 1997;23: 123-126.
8.- Seal DV, Hay J. Contact lens disinfection and Acanthamoeba: problems and practicalities. Pharm J 1992; 248:717–719.
9.- Bruinsma GM, Rustema-Abbing M, de Vries J, Stegenga B, van der Mei HC, van der Linden ML, Hooymans JM, Busscher HJ. Influence of wear and overwear on surface properties of etafilion. A contact lenses and adhesion of pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 2002;43:3646-3653.
10.- Jones L, Senchyna M, Glasier MA, Schickler J, Forbes I, Louie D, May C. Lysozyme and lipid deposition on silicone hydrogel contact lenses material. Eye Contact Lens. 2003;29(1 Suppl):S75-9.
11.- Subbaraman LN, Glasier MA, Senchyna M, Jones L. Stabilization of lysozyme mass extracted from lotrafilocone silicone hydrogel contact lenses. Optom Vis Sci 2005;82: 209-214.
12.- Vermeltfoort PB, Rustema-Abbing M, de Vries J, Bruinsma GM, Busscher HJ, van der Linden ML, Hooymans JM, van der Mei HC. Influence of day and night wear on surface properties of silicone hydrogel contact lenses and bacterial adhesion. Cornea. 2006; 25: 516-523.
13.- MA Subbaraman LN, Glasier MA, Senchyna M, Sheardown H, Jones L. Extraction efficiency of an extraction buffer used to quantify lysozyme deposition on conventional and silicone hydogel contact lens materials. Eye Contact Lens. 2007; 33: 169-173.
14.- Glasier MA, Keech A, Sheardown H, Subbaraman LN, Jones L. Conformational and quantitative characterization of lysozyme extracted from galyfilcon and senofilcon silicone hydrogel contact lenses. Curr Eye Res. 2008;33:1–11
15.- Carney FP, Nash WL, Sentell KB. The Adsorption of major tear film deposit in vitro to various silicone hydrogel over time. Invest ophthalmol Vis Sci. 2008;49: 120-124
16.- Santos L, Rodrigues D, Lira M, Real Oliveira ME, Oliveira R, Vilar EY, Azeredo J. Optom Vis Sci. 2008;85:520-525.
ACKNOWLEDGEMENT: The author is thankful to Gaymed Labs Pvt Ltd, Delhi, India for their financial and technical support.
Correspondence:
A. Arora.
B-264, Derawal Nagar, Opposite Model Town II.
Delhi - 11009, India.
alkamailin @ yahoo.co.in