Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HIGH-LEVEL AMINOGLYCOSIDE RESISTANCE ENTEROCOCCUS SPP IN A TERTIARY CARE HOSPITAL IN MEXICO.
José Arellano Galindo M. Sc.1, 2, Yazmín Garzón Tejada3, Silvia Giono Cerezo Ph. D. 2,
Olga Mateos Salazar3, Efrén Alberto Pichardo Reyes M.D.4
1Laboratorio de Trasplante de Médula Osea, Hospital Infantil de México Federico Gómez.
2Laboratorio de Bacteriología Médica, Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas IPN.
3 Laboratorio de Microbiología, Sección de Patología Clínica Hospital Central Militar Secretaría de la Defensa Nacional.
4 Hospital Central Militar, Secretaria de la Defensa Nacional.
México
Rev Electron Biomed / Electron J Biomed 2005;1:40-45
Comment of the reviewer, Prof. Jose María Eirós Bouza, MD. PhD. Professor of Microbiology. School of Medicine. University of Valladolid. España.
Comment of the reviewer Erhan Suleymanoglu PhD. Gazi Universitesi, Eczacilik Fakultesi, Department of Pharmaceutical Chemistry. Gazi Mahallesi, Ankara. Turkey
ABSTRACT
Enterococcus is one important cause hospital-acquired infections. High levels of resistance for aminoglycosides (HLAR) as gentamicin (HLGR) and streptomycin (HLSR) in Enterococcus isolates in a tertiary clinical care in Mexico City were studied.
Identified using Microscan® system. Resistance to ampicillin, streptomycin, gentamicin and vancomycin according to NCCLS. HLGR and HLSR were confirmed using disks. 91 strains were isolated and identified from clinical samples from January 1998 to January 1999. Two species were identified. 83 (91.2 %) E. faecalis and 8/91 (8.8 %) were E. faecium. E. faecalis in urine samples were 67/91 (73.6%). Neither showed vancomycin or ampicillin resistance; 1/8 E. faecium was ampicillin resistant. 30/83 (36%) E. faecalis and 3/8 E. faecium were gentamicin resistant; while 39/83 (47.0%) E. faecalis and 4/8(50%) E. faecium were streptomycin resistant. 14/83 (16%) E. faecalis, 3/8 E. faecium showed sensitive pattern for gentamicin and streptomycin. None strains were 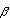 -lactamases producer. E. faecalis 12/83 (14.4%) were HLGR and 28/83 (33.7%) were HLSR. E. faecium. 2/8 were HLGR and 2/8 were HLSR. HLAR 33/83 (39.7%) were E. faecalis and 3/8(37.5%) were E. faecium isolated from urine. E. faecalis was more frequent than E. faecium and show that HLAR in Enterococci is high and could be a serious problem if spread as nosocomial infection.
-lactamases producer. E. faecalis 12/83 (14.4%) were HLGR and 28/83 (33.7%) were HLSR. E. faecium. 2/8 were HLGR and 2/8 were HLSR. HLAR 33/83 (39.7%) were E. faecalis and 3/8(37.5%) were E. faecium isolated from urine. E. faecalis was more frequent than E. faecium and show that HLAR in Enterococci is high and could be a serious problem if spread as nosocomial infection.
Keywords: Enterococcus, High level aminoglycoside resistance, HLAR, HLGR, HLSR.
RESUMEN:
Enterococcus es una causa importante de infección intrahospitalaria. Se determinaron los niveles altos de resistencia para aminoglucósidos(HLAR), gentamicina (HLGR) y estreptomicina (HLSR) en Enterococcus aislados de diversos casos clínicos en un hospital de tercer nivel en México, D.F.
La identificación se realizó usando el sistema de Microscan® y la resistencia a ampicilina, estreptomicina, gentamicina, vancomicina, HLGR y HLSR de acuerdo a la NCCLS. 91 cepas fueron aisladas de muestras clínicas de Enero de 1998 a Enero 1999, se identificaron dos especies. 83 (91.2%) E. faecalis y 8/91 (8.8%) fueron E. faecium. 67/91 (73.6%) E. faecalis se aislaron de muestras de orina. Ninguna cepa mostró resistencia a vancomicina; 1/8 E. faecium fue ampicilina resistente. 30/83 (36%) E. faecalis y 3/8 E. faecium fueron resistentes a gentamicina, mientras que 39/83 (47.0%) E. faecalis y 4/8(50%) E. faecium fueron resistentes a estreptomicina. Además 14/83(16%) E. faecalis y 3/8 E. faecium fueron sensibles para ambos aminoglucósidos (gentamicina y estreptomicina). Ninguna cepa fué productora de 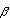 -lactamasas. 12/83 (14.4%) E. faecalis mostraron HLGR y 28/83 (33.7%) HLSR. 2/8 E. faecium fueron HLGR y 2/8 fueron HLSR. De los aislamientos de orina 33/83 (39.7%) E. faecalis fueron HLAR y 3/8(37.5%) E. faecium. E. faecalis fue más frecuente que E. faecium. Este trabajo muestra que Enterococcus ssp. HLAR se aisla y podría ser un problema en brotes de infección nosocomial.
.
-lactamasas. 12/83 (14.4%) E. faecalis mostraron HLGR y 28/83 (33.7%) HLSR. 2/8 E. faecium fueron HLGR y 2/8 fueron HLSR. De los aislamientos de orina 33/83 (39.7%) E. faecalis fueron HLAR y 3/8(37.5%) E. faecium. E. faecalis fue más frecuente que E. faecium. Este trabajo muestra que Enterococcus ssp. HLAR se aisla y podría ser un problema en brotes de infección nosocomial.
.
INTRODUCTION
Enterococci are normal inhabitants of intestinal tract of human and colonize oral and vaginal cavity1. Enterococcus can be found in soil, food, water, plants animals, birds and insects2, 3.
Infections can be found in elderly patients and immunocompromised hospitalized patients that had received antimicrobial therapy and others risk factors as the administration of immunosuppressive drugs, circulatory failure or clinical invasive procedures4-6.
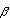 -lactamic or aminoglycoside monotherapy often results in clinical failure because Enterococci strains show intrinsic low-levels of resistance6. Therefore the treatment in a combination between
-lactamic or aminoglycoside monotherapy often results in clinical failure because Enterococci strains show intrinsic low-levels of resistance6. Therefore the treatment in a combination between 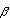 -lactamics and aminoglycosides is often used, but this combination can select high levels resistance aminoglycosides (HLRA) strains6, 7. Enterococci
-lactamics and aminoglycosides is often used, but this combination can select high levels resistance aminoglycosides (HLRA) strains6, 7. Enterococci 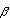 -lactamases producer is not observed but laboratory
-lactamases producer is not observed but laboratory 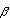 -lactamic pattern needs to be made on clinical samples8, sinergy between
-lactamic pattern needs to be made on clinical samples8, sinergy between 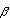 -lactamics and aminoglycosides most be predicted for enterococci by screening test specially streptomycin and gentamicin in order to predict high levels aminoglycosides resistance (HLAR) in Enterococcus strains9-12.
-lactamics and aminoglycosides most be predicted for enterococci by screening test specially streptomycin and gentamicin in order to predict high levels aminoglycosides resistance (HLAR) in Enterococcus strains9-12.
The increasing incidence of vancomycin resistance in enterococci strains (VRE) is necessary to report because the epidemiological impact at the hospital. VRE has been related with outbreaks in many centers in several countries13-17. High risk of vancomicin resistance has been observed in HLAR strains18.
HLAR and VRE had been report in Mexico for enterococci in hospitals where the use of these antibiotics are frequently19, 20. In this report we showed an analysis of the high levels of resistance for aminoglycosides and vancomycin resistance in Enterococcus isolates in a tertiary adults care in Mexico City.
MATERIAL AND METHODS
Clinical samples and control strains. Throughout a year, we obtained strains of Enterococci from several clinical samples of hospitalized and outpatients of Hospital Central Militar in Mexico City in 1998, strains were isolated and identified using Microscan® system and species identification was carry out as Faklam's criteria21. Controls strains were E. faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa 27853, E. coli 25922, Haemophilus influenzae ATCC 35540 and E. faecalis ATCC 51299.
Antimicrobial susceptibility test. All strains were tested with Kirby-Bauer disk diffusion method on Mueller-Hinton agar according to NCCLS using BBL® disks of 10 µg for ampicillin, streptomycin, gentamicin and 6 µg for vancomycin. Results were observed after incubation period at 35°C for 24-48 h22.
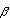 -lactamases detection. The
-lactamases detection. The  -lactamases producer strains were tested using cefinase disks (BBL®), according to the manufacturer.
-lactamases producer strains were tested using cefinase disks (BBL®), according to the manufacturer.
High levels aminoglycosides resistance test. HLGR and HLSR were screening for gentamicin and streptomycin using susceptibility test Microscan® with pos combo 4 I microplate dehydrated panels according to the manufacturer's and were confirmed using disks of HLRG and HLRS as describing by NCCLS22. Briefly, two or three colonies from a blood agar base (BAB) plus 5% sheep blood plate culture of 24-48 h at 35°C, were inoculated on brain heart infusion (BHI) and incubated 35°C until reach the 0.5 tube of Mac Farland and 10 10 ml from this suspension, Mueller-Hinton plates were inoculated22. BBL® disks were use to select high level resistance criteria, growth selection was observed with HLGR disk with 120 µg of gentamicin and 300 µg HLSR disk for streptomycin. The plates were incubated at 35°C 24 h and the size in mm of inhibition was measured. The results were evaluated according to NCCLS22.
RESULTS
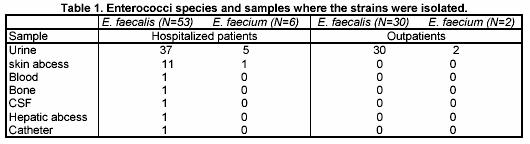
Clinical Isolation. A total of 91 strains were isolated and identified as Enterococcus spp, from several clinical samples from January 1998 to January 1999, at Clinical Pathology laboratory, Microbiology Department in Hospital Central Militar in Mexico City. Two species were identified, 83 (91.2%) strains were E. faecalis and 8 (8.8%) were E. faecium. Most of them, E. faecalis (67/91) and. E. faecium (7/8), were isolated from urine samples (table 1).
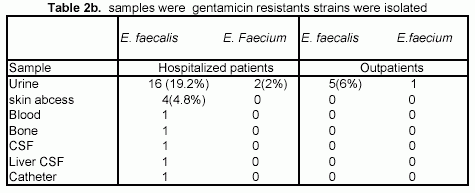
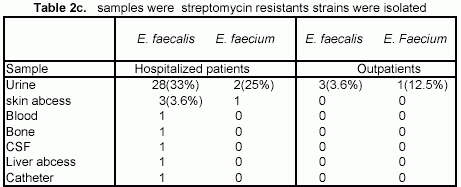
Antimicrobial resistance. None strains of E. faecalis showed vancomycin or ampicillin resistance, but only 1/8(12.5%) E. faecium strain, was ampicillin resistant. E. faecalis 30/83 (36%) were gentamicin resistant, which 21/30 (70%) from urine samples wich 16/30 (53.3%) of hospitalized patients and 5/30 (16.6%) were from outpatients. E. faecium gentamicin resistant were 2/8 from hospitalized patients and only 1 was from a outpatient disease 39/83 (47%) were streptomycin resistant, 31/39 (79.48%) were from urine. 28/39(70%) from hospitalized patients and 5/39(7%) from outpatients. E. faecium streptomycin resistant were 3/8 from hospitalized patients and only 1 was from a outpatient disease. (table 2a, 2b,2c).

 -lactamases production. None enterococci spp. strains were
-lactamases production. None enterococci spp. strains were  -lactamase producer.
-lactamase producer.
High level aminoglycoside resistance. E. faecalis 33/83 were HLAR, 12/83 (14.4%) were HLGR, 28/83 (33.7%) were HLSR. While E. faecium 3/8 HLAR, 2/8 (25%) showed HLGR and 2/8 HLSR (table 3). Wich E. faecalis 5/83 (6.0%) were HLGR and 21/83 (25%) were HLSR alone, 7/83 (8.4%) showed resistance for both gentamicin and streptomycin resistance. While E. faecium 1/8 (12.5%) showed HLGR and 1/8 (12.5%) HLSR alone, 1/8 (12.5%) were for both gentamicin and streptomycin resistance (figure 1).

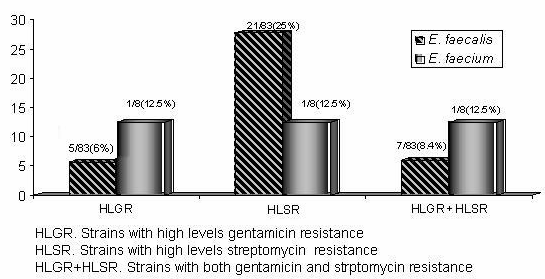
Figure 1.- Percentage obtained of strains HLSR, HLGR or HLSR+HLGR isolated on a year in a Tertiary clinical care
DISCUSSION
Antimicrobial resistance in Enterococcus has been increasing prevalence mainly in hospitalized patients23-25. Few studies have been performed in Mexico about the species and antimicrobial resistance. In 1996, Sifuentes-Osornio et al. 19, described the susceptibility in a tertiary-care adults center with a total of 407 enterococci strains identified. 162 from outpatients; 171 (42%) from urine as main isolate site. 325 (80%) were E. faecalis and 61 (15%) were E. faecium. As us no  -lactamases production were detected and 12% HLGR, all were susceptible vancomycin19. In a tertiary-care pediatric hospital in Mexico City 289 isolates, 38% from urine, 30% from catheter tips, 13.8% from surgical wounds, 11.8% from blood, 3.1% from peritoneal fluid, 2.1% from CSF and 3.3% from others showed E. faecalis 76.1%, E. avium 10%, E. faecium 5.2%, E. hirae 1.4%, E. malodoratus 1.4% and E. casseliflavus 0.6%. Ampicillin resistance were 29%, imipenem 17% and vancomycin 3%. None isolate had intermediate resistance to vancomycin. HLGR were 5.1% from 15 isolates, 6 were E. faecalis, 4 E. avium, 3 E. faecium and 2 E. casseliflavus. From 6 isolates HLSR none were resistant to vancomycin and one was
-lactamases production were detected and 12% HLGR, all were susceptible vancomycin19. In a tertiary-care pediatric hospital in Mexico City 289 isolates, 38% from urine, 30% from catheter tips, 13.8% from surgical wounds, 11.8% from blood, 3.1% from peritoneal fluid, 2.1% from CSF and 3.3% from others showed E. faecalis 76.1%, E. avium 10%, E. faecium 5.2%, E. hirae 1.4%, E. malodoratus 1.4% and E. casseliflavus 0.6%. Ampicillin resistance were 29%, imipenem 17% and vancomycin 3%. None isolate had intermediate resistance to vancomycin. HLGR were 5.1% from 15 isolates, 6 were E. faecalis, 4 E. avium, 3 E. faecium and 2 E. casseliflavus. From 6 isolates HLSR none were resistant to vancomycin and one was 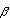 -hemolytic20.
-hemolytic20.
We detected in a year in a tertiary-care hospital 91 strains from different clinical samples from hospitalized 59/91 and outpatients 32/91, which were 83 (91.2%) E. faecalis and 8 (8.8%) E. faecium. The main site of isolation of strains was urine 67/83 E. faecalis and 7/8 E. faecium. These results were in accordance with others studies23-25. The emergence of enterococcus infection has related with urologic catheter in immunocompromised patients. Also Enterococcus have been implicated as nosocomial pathogen26.
All E. faecalis were ampicillin susceptible and only 1 E. faecium strain showed ampicillin resistance. Actually vancomycin resistance to enterococci (VRE) is investigated in several countries13-17, but in this none vancomycin resistance were observe. We suspected that vancomycin is restricted to use in this hospital. We did not observe none strain 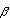 -lactamases producer neither E. faecalis nor E. faecium showed
-lactamases producer neither E. faecalis nor E. faecium showed 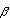 -lactamases detection that it was included as surveillance program.
-lactamases detection that it was included as surveillance program.
The rate of amynoglycosides resistance were gentamicin 21/83 (25%) E. faecalis and 3/8 (37.5%) E. faecium. Streptomycin 31/83 (37.3%) E. faecalis and 3/8 (37.5%) E. faecium were isolate from urine samples. Only 1/11 strains came from blood cultures; this was resistant for gentamicin and streptomycin (figure 1).
Overall E. faecalis HLGR were 12/30, HLSR was 28/40. E. faecium was in low frequency than E. faecalis but we can suppose more level of HLAR for E. faecium because despite the isolation were less than E. faecalis the HLAR percentage observed for E. faecium was higher (HLGR: 12/83, 14.45% vs 2/8, 25%; HLSR: 28/83, 33.73% vs 2/8, 25%).
Other authors has showed that HLAR can be two-folds higher in VRE isolates than in isolates of VSE18 but vancomicin resistance was not observe in these isolates, instead resistance for both aminoglycosides was observe. It is important to detect HLRG and HLRS because this would also help to limit the intrahospitalary dissemination of resistance and stablish a surveillance program about the use of vancomicin and aminoglycosides for management of enterococci infections.
This study show that HLAR in Enterococci is high and could be a serious problem in enterococci spread as nosocomial infection and HLGR is a possible risk and must be examinated in the antibiotic susceptibility pattern including HLAR detection and glycopetide screening.
REFERENCES
1. Murray B. The life and times of the Enterococcus. Clin Microbiol Rev 1990;1:46-65.
2. Ben O, Castro A, Lucas R, Abriouel H, Yousif N, Franz C, Holzapfel W, Perez-Pulido R, Martinez-Canamero M, Galvez A. Functional and safety aspects of Enterococci isolated from different Spanish foods. Syst Appl Microbiol. 2004 27:118-130.
3. Franzetti L, Pompei M, Scarpellini M, Galli A. Phenotypic and genotypic characterization of Enterococcus spp. of different origins. Curr Microbiol 2004 49:255-260.
4.Titze-de-Almeida R, Rollo Filho M, Eira CA, Rodriguez IP, Eudes-Filho J, Nascimento RS, Ferreira-li R, Moraes LM, Boelens H, Van Belkum A, Felipe MS. Molecular epidemiology and antimicrobial susceptibility of Enterococci recovered from Brazilian intensive care units. Braz J Infect Dis. 2004 8:197-205.
5. Khan MA, Siddiqui BK, Shamim A, Yosuf MA, Ahmed U, Zakiullah N, Burney IA. Emerging bacterial resistance patterns in febrile neutropenic patients: experience at a tertiary care hospital in Pakistan. J Pak Med Assoc 2004 54:357-360.
6. Levinson E, Sudhakar M. Increasing antimicrobial resistance: Therapeutic implications for Enterococcal infections. Curr infec Dis Rep 2000 2:417-423.
7. Haller M, Brandis M, Berner R. Antibiotic resistance of urinary tract pathogens and rationale for empirical intravenous therapy. Pediatr Nephrol 2004 19: 982-6.
8. Markowitz M, Wells V, Williams D, Stuart C, Coudron P, Wong E. Antimicrobial susceptibility and molecular epidemiology of B-lactamase-producing, aminoglycoside-resistant isolates of Enterococcus faecalis. Antimicrob Agents Chemother 1991 35:1075-1080.
9. Tsakris A, Pournaras S, Maniatis N, Douboyas J, Antoniadis A. Increasing prevalence of high-level gentamicin resistance among Enterococci isolated in Greece. Chemotherapy 2001 47:86-89.
10. Ronconi C, Merino L. Prevalence of Enterococcus faecalis and Enterococcus faecium with high resistance to aminoglycosides in the cities of Resistencia and Corrientes, Republic of Argentina. Enferm Infec Microbiol Clin 2000 18:271-273.
11. Zouain M, Araj F. Antimicrobial resistance of Enterococci in Lebanon. Int J Antimicrob Agents 2001 17:209-213.
12. Tejedor-Junco MT, Alonso-Rodriguez 0, Martin-Barrasa JL, Gonzalez-Martin M. Antimicrobial susceptibility of Enterococcus strains isolated from poultry faeces. Res Vet Sci 2005 78:33-38.
13. Cirak M, Sultan N. Prevalence of high level aminoglycoside and vancomycin resistance among enterococci in Turkey. Acta Microbiol Pol 1998 47:267-273.
14. Tambyah PA, Marx JA, Maki DG. Nosocomial infection with vancomycin-dependent enterococci. Emerg Infect Dis 2004 10:1277-1281.
15. Muratani T, Matsumoto T. Bacterial resistance to antimicrobials in urinary isolates. Int J Antimicrob Agents 2004; 24 SuppI 1:28-31.
16. Sanchez-Molina Ml, Martin D, Valladares C, Gastanares MJ, Torres C, Borque L. Susceptibility of Enterococcus genus to new antimicrobial agents Rev Esp Quimioter 2004 17:184-188.
17. Treitman A, Yarnold P, Warren J, Noskin G. Emerging incidence of Enterococcus faecium among hospital Isolates (1993 to 2002). J Clin Microbiol. 2005 42:462-4
18. Yazgi H., Ertek M., Erol S. and Ayyildiz A. A comparison of high-level aminoglycoside resistance in vancomycin-sensitive and vancomycin resistant Enterococcus species. J Int Med Res 2002 30:529-534.
19. Sifuentes-Osornio J, Ponce De León A, Munoz T, Villalobos Y, Ontiveros C, Gómez C. Antimicrobial susceptibility patterns and high level gentamicin resistance among enterococci isolated in a mexican tertiary care center. Rev Invest Clin 1996 48:91-96.
20 Miranda, G, Lee L, Kelly C, Solórzano F, Leaños B, Muñoz 0, Patterson J. Antimicrobial resistance from enterococci in a pediatric hospital. Plasmids in Enterococcus faecalis isolates with high-level gentamicin and streptomycin resistance. Arch Med Res 2001 32:159-163
21. Facklam R, Hollis D, Collins M. Identification of gram positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol 1989 4:724-30.
22. National Committee for Clinical Laboratory Standards: Methods for dilution antimicrobial tests for bacteria that grow aerobically; M7-A5. National Committee for Clinical Laboratory Standards, Wayne PA 52000, 2003
23. Udo E, Sweih A, Phillips A, Chugh T. Species prevalence and antibacterial resistance of enterococci isolated in Kuwait hospitals. J Med Microbiol 2003 52:163-168.
24. Molokacova M, Vaculikova A. Monitoring of antibiotic resistance in bacterial isolates from bacteremic patients. J Chemother. 2004 16:269-272.
25. 25. Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP. Emerging resistance among bacterial pathogens in the intensive care unit a European and North American Surveillance study (2000-2002). Ann Clin Microbiol Antimicrob(on line) 2004. Vol 3, No.14. Available: http://www.ann-clinmicrob.com/content/3/1/14. ISSN: 1476-0711.
26. Dworniczek E, Kuzko K, Mroz E, Wojciech L, Adamski R, Sobieszczanska B, Seniuk A. Virulence factors and in vitro adherence of Enterococcus strains to urinary catheters. Folia Microbiol 2003 48:671-678.
Acknowledments
We thanks to the clinical Pathology Service at the Hospital Central Militar and Laboratorio de Bacteriología Médica ENCB IPN by the facilities for performed this work. This work was supported by grant CGPI 2004753.
Corresponding author: Dra. Silvia Giono Cerezo.
Laboratorio de Bacteriología Médica, Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas IPN.
Apartado Postal CON-174 CP 06400 México DF.
E-mail: sgiono@yahoo.com. Tel. 57296300 ext. 62374.
enterococci are important nosocomial agents and serious infections caused by them are often treated with a combination of cell wall inhibitor and aminoglycoside. However, the presence of high level aminoglycoside resistance in these isolates makes this treatment combination ineffective. The prevalence of such isolates in a tertiary care set up has important diagnostic and therapeutic implications.
This study was conducted in a tertiary clinical care in Mexico City. The isolates were studied, identified using Microscan® system according to NCCLS, ampicillin, streptomycin, gentamicin and vancomycin. The number of clinical samples is low, newertheless the authors emphasizes the importance to make routine testing of the enterococcal isolates for high level aminoglycoside resistance. Alternative treatment regimens need to be sought, besides prudent use of antibiotics.
Comment of the reviewer, Prof. Jose María Eirós Bouza, MD. PhD.
Professor of Microbiology. School of Medicine. University of Valladolid. España.
