Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
LOW FRACTIONAL EXCRETION OF UREA IN HYPOTHYROIDISM INDUCED HYPONATREMIA
Musso CG, Macías Núñez JF*, Imperiali N, Algranati L.
Servicio de Nefrología - Hospital Italiano de Buenos Aires (Argentina) y
* Servicio de Nefrología - Hospital Universitario de Salamanca (España)
Rev Electron Biomed / Electron J Biomed 2005;1:64-67
Comment of the reviewer, Prof. Hélio Teixeira. Titular e libre docente. Departamento de Clínica Médica, Universidade Federal de Uberlândia, Uberlândia (MG), Brazil
Comment of the reviewer Prof. Jeyaraj Balasubramaniam. Resident Director. Kidney Care Centre. Tirunelveli. Tamilnadu, India.
RESUMEN:
El hipotiroidismo puede causar alteraciones del metabolismo del agua, los electrolitos, la hemodinamia e histología renales, siendo la hiponatremia y la reducción del filtrado glomerular sus consecuencias más significativas, pero poco prevalentes. Todos estos cambios son corregibles con el suministro de hormona tiroidea exógena.
La excreción fraccional de urea (EFU) es un índice útil en la evaluación de la hiponatremia, pero no se ha descripto aun el valor que este índice alcanza en la hiponatremia inducida por hipotiroidismo. En el presente reporte mostramos que la EFU y excreción fraccional de sodio (EFNa) fueron baja (EFU: 29%) y alta (EFNa: 2.2%) respectivamente en un paciente que padecía hipotiroideo severo. El tratamiento con hormona tiroidea normalizó el valor de ambos índices.
ABSTRACT
Hypothyroidism can cause disturbance of renal hemodinamics, kidney histology, water and electrolyte metabolism, being hyponatremia and glomerular filtration reduction their low prevalent but most significant consequences. All these changes are largely corrected by substitution of exogenous thyroid hormone.
Fractional excretion of urea (FEU) is a useful index in the evaluation of hyponatremia. However, it was not still reported in the literature the FEU value in hyponatremia induced by hypothyroidism. Because of that we presented a case report showing that the value of FEU and fractional excretion of sodium (FENa) were low (FEU: 29%) and high (FENa: 2.2 %) respectively in a severe hypothyroid patient. Treatment based on thyroid hormone normalized both indeces.
INTRODUCTION
Hypothyroidism can cause disturbance of renal hemodinamics, kidney histology, water and electrolyte metabolism, being hyponatremia and glomerular filtration reduction their most significant consequences. All these changes are largely corrected by substitution of exogenous thyroid hormone1.
One of the main points in the evaluation of hyponatremia is to determine the state of the extracellular volume (ECV). However, this evaluation is not always easy to perform clinically, specially to distinguish a normal ECV from a modestly contracted one2.
Fractional excretion of urea (FEU) is a useful index in the evaluation of hyponatremia and it is expected to be high (FEU > 65%) in hyponatremia with normal ECV, and low (FEU < 35%) in renal hypoperfusion states such as hyponatremia with low or high ECV3.
However, it is not reported in the literature how is the FEU in hyponatremia induced by hypothyroidism. Because of that we presented a case report describing the value of FEU in this entity and analyzing its physiopathological bases.
Case Report
A seventy seven- year-old man was referred to our nephrology department to investigate renal failure deterioration: plasma creatinine 2 mg/dl (normal in male: 0.6-1.3 mg/dl) and hyponatremia: plasma sodium 133 mmol/l (normal: 135-145 mmol/l). This patient had a previous history of:
1) Gout currently treated with allopurinol 300 mg/day
2) Hypertension treated with low sodium diet and atenolol 50 mg/day.
3) Aortic biological valvular replacement four years before
During the first encounter the patient presented many signs and symptoms compatibles with hypothyroidism such as increased tiredness, edematous non-elastic and dry skin, hoarse voice, low speech pattern and loss of lateral eyebrows. All these findings were long dating.
Biochemistry results included high plasma thyrotrofin (TSH): 100 IU/ml (normal up to: 4 IU/ml), normal glucose, total cholesterol, triglycerides, albumin, hemoglobin and creatinine kinase. Antithyroglobulin antibodies were negative.Urinalysis showed no proteinuria or hematuria, and urine sediment contained no cast. Thyroid ultrasound showed no goiter.
After hypothyroidism was confirmed, hormonal replacement was started. Initially, a low thyroxin hormone dose was prescribed (0.25 mcg/day), being this dose progressively increased until the patient was on 150 mcg/day six months later. At that moment the patient had normalized most of his previous physical signs and symptoms of hypothyroidism, plasma sodium (137 mmol/l) and TSH levels (4 IU/ml). A significant improvement in his renal failure was documented: new plasma creatinine 1.2 mg/dl.
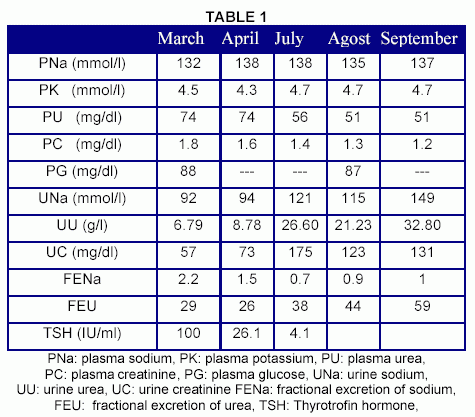
Since before and after starting the hormonal substitution urea, creatinine, sodium, and potassium were measured in plasma and urine samples, fractional excretion of these substances were calculated. It was observed that before the thyroxin prescription our patient had a low FEU (29%) and a high FENa (2.2%), while after the hormonal substitution there was an increase in the FEU and a reduction in the FENa respect to the initial values (Table 1)
DISCUSSION
At least one of the following components is needed to develop hyponatremia: a positive balance of free water or a negative balance of sodium 4.
Hypothyroidism induced hyponatremia is classically described as a disturbance secondary to a mechanism alike to the syndrome of inappropiate antidiuretic hormone secretion (SIADH)5.Some authors consider that the bases of hypothyroidism induced hyponatremia have not been yet understood6, in fact there are several facts that do not support this hyponatremia as secondary to a SIADH-like mechanism. Let is have a look at them:
1) The syndrome of inappropiate antidiuretic hormone secretion characteristicaly has an increased urea excretion. Even more, it was proposed that urea could be secreted in this syndrome since many patients suffering from SIADH have FEU above 100%7. Conversely, a reduced urea excretion was documented in hypothyroid rats 8. Moreover, in our hypothyroid patient the FEU before starting hormonal replacement was low (FEU: 29%), but it became normal after hormonal substitution was initiated (FEU: 59%) (Table 1). This could be interpretated as a diminution of the hypoperfusion state after thyroid hormone restitution due to a decrease in the intrarenal vasoconstriction and an increase in sodium reabsortion capabiliy.
2) Patients suffering from SIADH are expected to have high plasma vasopressin level2. However, not all the trials showed plasma vasopressin elevated in patients suffering from hypothyroidism induced hyponatremia 9.
3) The efficiency of vasopressin is reduced in hypothyroid patients due to a diminished concentration of medullary solutes, leading to urinary concentration deficit and polyuria10.
This phenomenon was attributed to a dysfunction in sodium reabsorbtion in the ascending loop of Henle8. Even more, studies performed in hypothyroid animal models have demonstrated low levels of aquaporin channels and vasopressin receptors V2 in the collecting tubules8,11.
All the above data are against a SIADH-like inducing mechanism of hyponatremia in hypothyroidism. Two other mechanisms appear to be crucial in the induction of hyponatremia secondary to hypothyroidism: an altered free water clearance and a negative sodium balance. On one hand, low free water clearance can be caused by a reduced solute delivery to the diluting nephron segments10,12. This phenomenon could be secondary to the low cardiac output, reduced glomerular filtration rate and renal vasoconstriction usually present in hypothyroid patients8,12,13. However, the trend to high proximal sodium loss suffered by these patients could diminish the impact of this mechanism. Besides, there is a trial that have demonstrated a dysfunction in the sodium reabsortion in the ascending loop of Henle, situation that affects the production of free water clearance since this is the tubular area where it is generated8. Although, other study in rats found no changes in the number of sodium luminal transporters of this nephron segment, this does not mean that their function is preserved11.
On the other hand, there is an increase in sodium loss in hypothyroid patients14.
It was reported that rats receiving antithyroid drugs together with a sodium-deficient diet were rapidly dying from negative sodium balance. One of the mechanisms resulting in the reabsorptive defect was suggested to be the dependency of tubular sodium reabsorption on the activity of Na-K-ATPase in proximal and collecting tubules which is known to be reduced in hypothyroid rats. Nevertheless, it has been demonstrated that the reduced activity of Na-K-ATPase alone can not account for the transport changes observed in hypothyroidism. The influence of thyroid hormone in the two major sodium transporters of the proximal tubule: type 3 Na/H exchanger and type 2 Na-Phosphate cotransporter has shown that both protein are directly regulated by this hormone8,11,14.
In addition, thyroid hormone has a permissive role on the action of aldosterone. This is another reason that explains the reduced sodium reabsorption capability in hypothyroid states11.
In our patient we observed that when he was suffering from hypothyroidism his FENa was high (FENa: 2.2), even he was on a low sodium diet. After starting hormonal replacement this index showed lower values (FENa: 0.9). This could be interpretated as a recovery of the tubular sodium reabsortion capability after thyroid hormone prescription.
Our patient was also an old man, and it has been described that in aged people there is a tendency to tubular sodium loss15, However, since his sodium loss resolved after thyroid hormone prescription this phenomenon seemed to be related to the endocrinological disturbance.
Even though, there is an important urinary sodium loss and contracted intravascular space in hypothyroidism, total body sodium content is increased and specially redistributed mostly to the the intersticial space9. This is due to the fact that hypothyroid patients have a leak of proteins (albumin) from the intravascular space to the intersticial one, and at the same time they have a low removal of proteins from the intersticial space by the linfatic system. Consequentely, there is an increase in the oncotic pressure in the intersticial compartment that attracts water and sodium locally resulting in the characteristic non-pitting edema called mixedema16.
As a consequence, all the above mentioned physiological modifications lead to an increase urinary sodium loss, reduced intravascular volume and expanded intersticial space explaining the combined pattern of high fractional excretion of sodium and low fractional excretion of urea of the hypothyroid hyponatremia.
CONCLUSION:
Fractional excretion of urea has low values in hypothyroidism induced hyponatremia. An altered free water clearance and sodium loss could be the main generating hyponatremia mechanism in hypothyroid patients.
REFERENCES
-
1.- Makino Y, Fujii T, Kuroda S, Inenaga T, Kawano Y, Takishita S. Exacerbation of renal failure due to hypothyroidism in a patient with isquemia nephropathy. Nephron 2000; 84: 267-269.
2.- Kokko JP. Disorders of fluid volume, electrolyte, and acid-base balance. In Bennet C, Plum F (Eds). Cecil Textbook of Medicine. Philadelphia. W.B. Saunders Company.1996: 534-537
3.- Bazerque F. 'Hypoosmolar and hyperosmolar syndroms'. In Pacin J, Dubin A, Gallesio A, Laffaire E, Maskin B, San Roman E (Eds). 'Intensive Care'. Buenos Aires. Panamericana. 1995: 369-384.
4.- Halperin ML, Goldstein MB. Hyponatremia. In Halperin ML, Goldstein MB (Eds). Fluid, electrolyte, and acid-base physioloy. Philadelphia. W.B. Saunders Company. 1999: 283-328.
5.- Dillmann W. The thyroid. In Bennet C, Plum F (Eds). Cecil Textbook of Medicine. Philadelphia. W.B. Saunders Company.1996:1237-1239
6.- Rose BD, Post TW. Hyposmolar syndromes. In Rose BD, Post TW (Eds). Clinical physiology of acid-base and electrolyte disorders. Madrid. Marbán. 2002: 710
7.- Bankir L. Urea and the kidney. In Brenner BM (Ed). The kidney. Philadelphia. W.B. Saunders company.1996: 571-606
8.- Cadnapaphornchai MA, Kim YW, Gurevich AK, Summer SN, Falk S, Thurman JM, Schrier R. Urinary concentrating defect in hypothyroid rats: role of sodium, potasium, 2-chloride co-transporter, and aquaporins. J Am Soc Nephrol 14: 566-574, 2003
9.- Wooddrow G, Brownjohn AM, Turney H. Acute-on-chronic renal failure and hyponatrremia associated with severe hyponatremia. Nephrol Dial Transplant 8: 557-559, 1993
10.- Rossi N, Schrier R. Hyponatremia. In Maxwell MH, Kleeman CR, Narins RG. (Eds). Clinical disorders of fluid and electrolyte metabolism. Buenos Aires. Panamericana. 1991: 417
11.- Schmitt R, Klussmann E, Kahl T, Ellison D, Bachmann S. Renal expression of sodium transporters and aquaporin-2 in hypothyroid rats. Am J Physiol 284: F1097-1104. 2003
12.- Nakahama H, Sakaguchi K, Horita Y, Sasaki O, Nakamura S, Inenaga T, Takishita S. Treatment of severe hypothyroidism reduced serum creatinine levels in two chronic renal failure patients Nephron 2001; 88: 264-267
13.- Kreisman S, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med 1999; 159: 79-82
14.- Shah M, Quigley R, Baum M. Maturation of proximal straight tubule NaCl transport: role of thyroid hormone. Am J Physiol Renal Physiol 278: F596-F602, 2000
15.- Musso C. Geriatric nephrology and the "nephrogeriatric giants". International Urology and nephrology 34:255-256, 2002
16.- Rose BD, Post TW. Edematous states. In Rose BD, Post TW (Eds). Clinical physiology of acid-base and electrolyte disorders. Madrid. Marbán. 2002: 484
