Indice del volumen
Volume index
Comité Editorial
Editorial Board
Comité Científico
Scientific Committee
GENOTYPES OF THE HELICOBACTER PYLORI ISOLATES AND THE IL-1 GENES IN KAZAN CITIZENS (KAZAN, RUSSIA) WITH GASTRIC AND DUODENAL ULCER
Olga A. Chernova1, Elmira R. Nasybullina1, Vladislav M. Chernov1,
Oleg V. Gorshkov1, Gulnara F. Shaimordanova1, Rustem A. Abdulhakov2,
Maxim V. Trushin1*
1Kazan Institute of Biochemistry and Biophysics, Kazan, Russia
2Kazan State Medical University, Kazan, Russia
mtrushin @ mail.ru
Rev Electron Biomed / Electron J Biomed 2006;1:13-18
Comment of the reviewer Angel San Miguel, MD. PhD Servcio de Análisis Clínicos. Hospital Universitario Rio Hortega. Valladolid. España
Comment of the reviewer Erhan Süleymanoglu PhD. G.U.E.F., Department of Pharmaceutical Chemistry, Gazi University. Gazi Mahallesi, Ankara. Turkey
ABSTRACT
The prevalence of the virulence genes (iceA, cagA, babA2, vacA) in the Helicobacter pylori strains isolated from patients with clinic and histologically proved diagnosis of gastric and duodenal ulcer as well as the distinction of the IL-1 genes in the helicobacter carriers were studied. VacA s1/m2 iceA1 cagA+ and vacA s1/m2 iceA1 cagA+ babA2 genotypes of H. pylori were shown to be the prevalent in the clinic isolates. Four patients were found to be infected with Ureaplasma urealyticum. The combination of the IL-1B-511*?/IL-1B+3954*C/IL-1RN*2 alleles was shown to be the prevalent among the patients with gastric and duodenal ulcer. The correlation between the H. pylori genotypes, the IL-1 genes and the ulcer particularity was not found.
Key words: Helicobacter pylori, virulence genes, IL-1 genes, genotyping, ulcer disease, persistence, Ureaplasma urealyticum
INTRODUCTION
An expressed genetic heterogeneity of the Helicobacter pylori strains determines the distinct clinical implications of the infection. In most of the H. pylori-infected people, clinical presentations are absent. In some individuals, however, colonization of the stomach mucous tunic with this bacterium may be the possible reason for development of chronic gastritis, peptic ulcer as well as gastric adenocarcinoma and B-cell lymphoma1.
Pathogenicity of H. pylori is connected with products of the ureI, iceA, cagA, babA2 as well as vacA genes2. Distribution of the H. pylori genotypes depends on the ethnogeographic peculiarities3. Moreover, polypathia (H. pylori+Ureaplasma urealyticum infection) may modify the clinical aspects of the infection4.
It is known that pathogenesis depends on the biology of the pathogen, the interaction between the infective agent and the defense systems of the host5.
Recently, it was found that genetic predilection to various infections depends on the polymorphism of the genes for interleukins6, 7. Furthermore, allelic variability in IL-1 genes may be the reason for the distinct susceptibility to the H. pylori infection8.
The subject of the study was to elucidate the genotypes of the Helicobacter pylori isolates and the IL-1 genes in Kazan citizens (Kazan, Russia) with gastric and duodenal ulcer.
MATERIALS AND METHODS
We obtained gastric biopsy specimens from 21 H. pylori-infected patients (20-78 years old citizens of Kazan, Russia) with gastric and duodenal ulcer. Biopsy and its analysis were performed as described by Momynaliev and co-workers9. For the extraction and purification of DNA, "HelicoPol" reagents (Litech Corporation, Moscow, Russia) were used. H. pylori and U. urealyticum were revealed by PCR assay. Genotyping of the cagA, iceA and babA2 genes was performed with the specific reagents of Litech Corporation (Moscow, Russia). The results were documented using the "DNA Analyzer" vision system.
PCR analysis of multiform locuses for IL-1Ra and IL-1b genes (at minus 511 and plus 3954 position) was performed with a programmable thermal cycler "Tercyc" (DNA-technology, Russia). Variants of C-511T and C+3954T for IL-1B and VNTR for IL-1RN (alleles 1 and 2) genes were determined as described by Garcia-Gonzales and co-workers8. The amplified regions were treated with AvaI (C511T) and TaqI (C+3954T) restrictases (Fermentas, Lithuania). The lengths of the restriction fragments were as follows: 135 and 114 b.p. (presence of the restriction site, allele C) and 249 b.p. (absence of the restriction site, allele T) for TaqI; 190 and 115 b.p. (presence of the restriction site, allele C) and 305 b.p. (absence of the restriction site, allele T) for AvaI. The length of IL-1RN*1 (IL-1Ra allele 1) was 410 b.p., and for IL-1RN*2 (IL-1Ra allele 2) was 240 b.p.
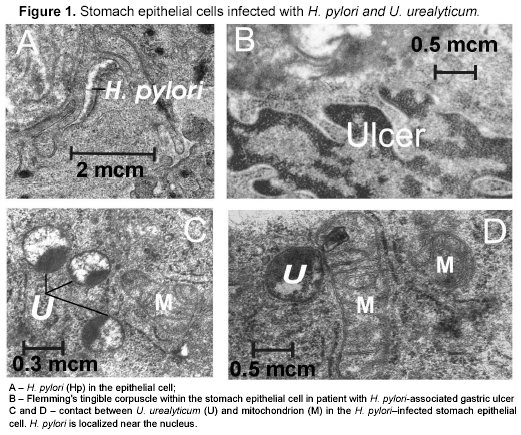
The electron-microscopic analysis was performed with the use of Hitachi HU-125 transmissible device (Hitachi, Japan).
The data were analyzed using the  2 test implemented in a commercially available computer program. A value of P<0.05 was considered significant.
2 test implemented in a commercially available computer program. A value of P<0.05 was considered significant.
RESULTS AND DISCUSSION
Helicobacters (H. pylori) were revealed in all patients. Moreover, U. urealyticum was also detected in 4 patients (19%). Due to genotyping of vacA gene, both s1 and s2 alleles were identified in two samples from people with duodenal ulcer. Two distinct strains of H. pylori were possibly the reason for this finding. In order to avoid difficulties in interpretation of the results, these two samples were eliminated from the analysis.
Urease is the universal factor of H. pylori pathogenicity10. Products of the cagA, vacA (s1, s2, m1, and m2), iceA (A1, A2) and babA (A1, A2) genes are believed to contribute an additional input into H. pylori pathogenicity and clinical presentation2, 11.
cagA+ isolates of H. pylori were obtained in 14 cases (73.7%) out of the remaining 19 biopsy specimens. This frequency of cagA+ is somewhat lower than that of observed in Europe and Central Russia9. High frequency variability of the gene as well as alterability in genetically determined susceptibility of the host organism to various H. pylori strains12 is probably the explanation for the above-mentioned fact. The presence of the cag and vac pathogenicity islets in the H. pylori strains is believed to prevent the englobing of these bacteria and favors their persistence13.
All the cagA+ H. pylori strains contained vacA s1 allele. It should be noted that distribution of the vacA alleles is different in various ethnopopulations. However, infection with vacA s1/m1 H. pylori is associated with more defined inflammation. H. pylori strains with vacA s1/m1 and vacA s1/m2 has maximal or median level of cytotoxin secretion while the vacA s2/m2 has not14. Indeed, vacA s1/m2 genotypes were revealed in 94.7% ulcer cases regardless of the disease location.
babA2 gene is thought to be a marker for duodenal ulcer and adenocarcinoma of stomach15. In our case, this gene was determined in 8 (42%) clinical isolates of H. pylori.
Infiltration of the stomach mucous membrane with polymorphonuclear leukocytes is more expressed in iceA1 H. pylori-infected people11. However, there is no a well-defined link between development of ulcer disease and presence of the iceA1 gene11, 16. We revealed iceA1 gene in 73.7% of the investigated patients while the iceA2 gene was not determined at all.
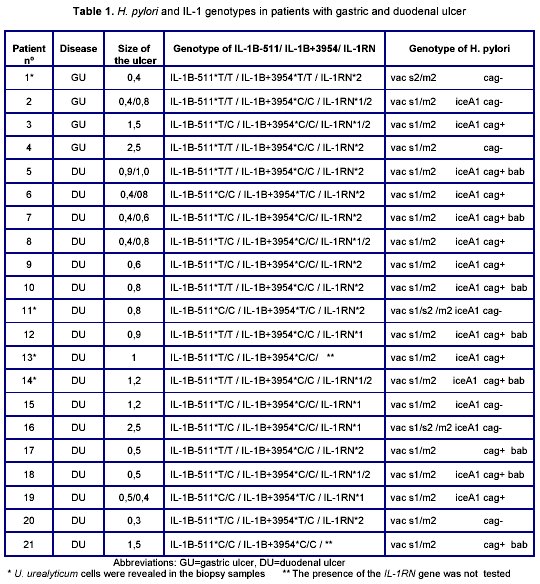
K. Momynaliev and co-workers9 suggested a hypothesis about the leading role of combination of the pathogenicity factors in development of the H. pylori infection. Thus, in general, H. pylori have about 28 genotypes. In our study, 6 genotypes of H. pylori were revealed (Table 1). Previously, we did not obtain any correlation between combination of the revealed genotypes and ulcer location17.
As far as it is known infection with bacteria and/or tissue damage result in activation of IL-1 expression. IL-1 family includes two agonists (IL-1A and IL-1B) and antagonist IL-1Ra. The susceptibility of individuals to some pathology might be connected with allele combination of the IL-1b and IL-1Ra genes6,7.
Distribution of the IL-1 genes (IL-1B-511, IL-1B+3954, IL-1RN) in patients with gastric and duodenal ulcer is presented in Table 1. It is clear from the Table 1 that the combination of the following alleles was the most frequent: IL-1B-511*T/ IL-1B+3954*C/IL-1RN*2.
According to the data of Garcia-Ganzales et al., combination of IL-1B-31*T/IL-1B-511*T/ IL-1B+3954*C/ IL-1RN*2 is important for duodenal ulcer progression in Europeans8. The data differences concerning the IL-1B-511 might be connected with ethnogeographic aspects. It is likely that othe factors including polypathia (in particularly, H. pylori+U. urealyticum infection) may promote to ulcer progression in people with other combination of IL-1 alleles (Table 1, Fig 1).
We could not observe a well-defined correlation between specific H. pylori genotypes and features of ulcer disease. This may point out the presence of additional factors promoting H. pylori virulence and connected with the peculiarities of microbiota in the pathology seat as well as with polymorphism of various genes determining the specific and the nonspecific signal pathways of the "parasite-host" system.
REFERENCES
-
1.Cover TL, Blasser MJ. Helicobacter pylori factors associated with disease. Gastroenterology 1999; 117: 257-261.
2. Ge Z., Taylor DE. Contribution of genome sequencing to understanding the biology of Helicobacter pylori. Annu Rev Microbiol 1999; 53: 353-387.
3. Andreson H, Loivukene K, Sillakivi T, Maaroos HI, Ustav M, Peetsalu A, Mikelsaar M. Association of cagA and vacA genotypes of Helicobacter pylori with gastric diseases in Estonia. J Clin Microbiol. 2002; 40: 298-300.
4. Abdulhakov RA, Chernova OA, Nasybullina ER, Chernov VM. Helicobacter pylori infection: synergism, genotypes, polymorphism and immunoresponsiveness. Pediatria 2002; 2: 19-21. In Russian.
5. Roitt IM, Brostoff J, Male DK Immunology, Moscow: Mir, 2000. In Russian
6. Freidin MB, Puzyrev VP, Ogorodova LM, Kobiakova OS, Kulmanakova IM. Polymorphism of interleukins and interleukin receptor genes: population distribution and association with atopic bronchial asthma. Genetika 2002; 38: 1710-1718. In Russian.
7. Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Alves CC, Campos ML, van Doorn L-J, Caldas C, Seruca R, Carneiro F, Sobrinho-Simoês M. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology 2003; 125: 364-371.
8. Garcia-Gonzalez MA, Lanas A, Savelkoul PHM, Santolaria S, Benito R, Crusius JBA, Peña AS. Association of interleukin 1 gene family polymorphisms with duodenal ulcer disease. Clin Exp Immunol 2003; 134: 525-531.
9. Momynaliev K, Smirnova O, Kudryavtseva L, Govorun V. Helicobacter pylori genotypes in Russia. Eur J Clin Microbiol Infec Dis 2003; 22: 573-584.
10. Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol 2000; 165: 1918-1924.
11. Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol 1999; 37: 2274-2279.
12. Yamaoka Y, Kodama T, Kashima K, Graham DY, Sepulveda AR. Variants of the 3'region of the cagA gene in Helicobacter pylori isolates from patients with different H.pylori-associated diseases. J Clin Microbiol 1998; 36: 2258-2263.
13. Shkitin VA, Shpirna AI, Starovoitov GN. The role of Helicobacter pylori in human pathology. Clin Microbial Antimicrobial Ther 2002; 4: 128-145. In Russian.
14. van Doorn L-J, Figueiredo C, Sanna R, Pena S. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol 1998; 36: 2597-2603.
15. Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A 1999; 96:12778-12783.
16. Graham DY, Yamaoka Y. Disease-specific Helicobacter pylori virulence factors: the unfulfilled promise. Helicobacter 2000; 5 Suppl 1: S3-9; discussion S27-31.
17. Nasybullina ER, Abdulhakov RA, Chernova OA, Gorshkov OV, Chernov VM. Distribution of Helicobacter pylori genotypes among the patients with gastro-duodenal disease. Exp Clin Gastroenterol 2004; 1: 126-132. In Russian.
We think that the article under consideration it is a good work. This contribution concerns to the Helicobacter pylori infection as cause of chronic superficial gastritis which, in same cases, will progress to peptic ulceration, and gastric carcinoma. This bacteria shows a very important genetic diversity. Nevertheless the genotypes involved, it has been demonstrated that successful treatment of H. pylori infection results in the cure of peptic ulcer, and the prevention of more severe diseases. Recently, it has been also demonstrated that the emergence of resistant strains to the antimicrobial agents of common clinical use are not only due to pinpoint mutations, but also to deletion of nucleotidic sequences, and to insertion of transposons1-6.
Several authors have show the prevalence of the virulence genes (iceA, cagA, babA2, vacA) in the Helicobacter pylori strains isolated from patients with clinic and histologically proved diagnosis of gastric and duodenal ulcer5-9. The same authors have elucidated the presence or absence of Il-1 genes. Also, have studied the H. pylori VacA s1/m2 iceA1 cagA+ and vacA s1/m2 iceA1 cagA+ babA2 genotypes, and the different isolated of clinical simples.
They also have found that the combination of the IL-1B-511*?/IL-1B+3954*C/IL-1RN*2 alleles is prevalent among the patients with gastric and duodenal ulcer. But any the correlation between the H. pylori genotypes, the IL-1 genes and the ulcer particularity was not found7-10. Finally, in basis of above consideration; I recommend the publication of the article.
References:
The role of the proinflammatory cytokine interleukin-1 (IL-1) in host susceptibility to Helicobacter pylori-associated gastric pathophysiology, inflammation and carcinogenesis is well-established. Recent data suggest that this susceptibility may be under genetic control. The presence of highly prevalent genetic polymorphisms provided for an ideal opportunity to design the appropriate epidemiologic studies to test for the role of potential candidate loci.
Since H. pylori achieves most of its damage through induction of chronic inflammation, it is worth considering candidate interleukin genes that control this process. Thus, functional polymorphism of IL-1 gene have been related to various risk factors of gastric cancer and duodenal ulcer. However, their importance in gastric ulcer remains elusive. To clarify the possible association between gastric and duodenal ulcer and the polymorphism in the IL-1, the authors studied the genotypes of H. pylori isolates from 21 patients in Kazan, Russia. The biopsy specimens were followed with PCR assay and electron microscopy. Genotyping revealed the prevalence of IL-1B-511*T/IL-1B+3954*C/IL-1RN*2 allele combination. Since different loci are reported by others in a similar studies, it appears that the variations are due to ethnogeographic aspects.
Therefore, the presented study reporting genetic data from Tatarstan, Russia can be regarded as an interesting contribution to previous epidemiological studies. The lack of correlation between the reported genotypes and disease parameters is also supported by the findings of other groups, implying that apparently the relationships among IL-1 gene polymorphism, the presence of H. pylori infection, and disease outcome are more complex than initially proposed. The present work undobtedly is a valuable addition to more detailed studies of the IL-1 gene cluster needed, as well as to its role in H. pylori determined gastric pathogenesis.
* Corresponding author:
Dr. Maxim V Trushin,
Received, January 12, 2006.
Comment of the reviewer Angel San Miguel, MD. PhD Servcio de Análisis Clínicos. Hospital Universitario Rio Hortega. Valladolid. España
1. Garza-González E, Pérez-Pérez GI, Tijerina-Menchaca R, Maldonado-Garza HJ, Bosques-Padilla FJ. Genotipos de Helicobacter pylori y su asociación con la respuesta inmune del hospedero. Rev Gastroenterol Mex 2002; 67: 155-160.
2. Ando T, Peek R. M., Pride D, Levine S. M, et al. Polymorphisms of Helicobacter pylori HP0638 Reflect geographic origin and correlate with cagA status. J Clin Microbiol 2002; 40: 239-246.
3. Atherton J, Cao P, Peek R, Tummuru M, Blaser M, Cover T. 1995. Mosaicism in Vacuolating Cytotoxin Alleles of Helicobacter pylori. J Biol Chem. 270: 17771-17777.
4. Atherton J, Peek R, Tham K, Cover T, Blaser M. 1997. Clinical and Pathological Importance of Heterogeneity In vacA, The Vacuolating Cytotoxin Gene of Helicobacter pylori. Gastroenterol. 112: 92-99.
5. Atherton JC. 1997. The Clinical Relevance of Strains Types of Helicobacter pylori. Gut 40:701-703.
6. Harris PR, Cover TL, Crowe DR, Orenstein JM, Graham MF, Blaser MJ, Smith PD. Helicobacter pylori Cytotoxin Induces Vacuolation of Primary Human Mucosal Epithelial Cell. Infec Immun. 1996; 64: 4867-4871.
7. Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA Gene from cagA-Positive Strains of Helicobacter pylori isolated in Japan. J Clin Microbiol 1994; 35: 1710-1714.
8. Garcia-Gonzalez MA, Lanas A, Savelkoul PHM, Santolaria S, Benito R, Crusius JBA, Peña AS. Association of interleukin 1 gene family polymorphisms with duodenal ulcer disease. Clin Exp Immunol 2003; 134: 525-531.
9. Wang H, Kuo C, Yeh A, Chang P, Wang W. Vacuolating Toxin Production in Clinical Isolates of Helicobacter pylori with Different vacA Genotypes. J Infect Dis. 1998; 178: 207-212.
10. van Doorn LJ, Schneeberger PM, Nouhan N, Plaisier AP, Quint WGV, De Boer WA. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut. 2000; 46:321-326.
Comment of the reviewer Erhan Süleymanoglu PhD. G.U.E.F., Department of Pharmaceutical Chemistry, Gazi University. Gazi Mahallesi, Ankara. Turkey
Kazan Institute of Biochemistry and Biophysics, PO BOX 30, Kazan 420111, Russia
Mail: mtrushin @ mail.ru
Published, January 30, 2006.
