Indice del volumen
Volume index
Comité Editorial
Editorial Board
Comité Científico
Scientific Committee
MEDICAL HYPOTHESES
SOME NEUROCHEMICAL DISTURBANCES IN MULTIPLE SCLEROSIS
Vladimir V. Markelov1 and Maxim V. Trushin 2,3*
1Kazan Municipal Rehabilitation Medical Health Center "Sanatorium Krutushka"
2Kazan Institute of Biochemistry and Biophysics, 3 Kazan State University, Department of Genetics. Kazan, Russia
mtrushin @ mail.ru
Rev Electron Biomed / Electron J Biomed 2006;2:46-54.
Comment of the reviewer José María Trejo Gabriel y Galán MD. PhD. Head of Neurology. Hospital General Yagüe. Burgos. Spain
Comment of the reviewer Carlos Musso MD. Hospital Italiano. Buenos Aires. Argentina
ABSTRACT
The data presented in this manuscript suggest a pivotal role of the central nervous system (CNS) in the regulation of immune status. We describe here that some neurochemical disturbances may provoke development of various diseases including multiple sclerosis. Some theoretic and practical backgrounds, how to improve the multiple sclerosis sufferers and patients with other autoimmune disorders, are also given.
Keywords: Multiple sclerosis, neurotransmitters, tryptophan, immunomodulation.
RESUMEN
Los datos que presentamos en este manuscrito, sugieren un papel guia del sistema nervioso central (SNC) en la regulación del estado inmune. Describimos aquí que varias alteraciones neuroquímicas pueden provocar el desarrollo de varias enfermedades, incluyendo esclerosis múltiple. También se comenta acerca del trasfondo teórico y práctico, y cómo mejorar a víctimas y pacientes con esclerosis múltiple y otras alteraciones autoinmunes.
Palabras clave: Esclerosis múltiple, neurotransmisores, triptofano, inmunomodulación.
INTRODUCTION
A bulk of studies devoted to multiple sclerosis (MS) is concentrated on therapy of the disease while works on the MS pathogenesis are not so numerous. But even herewith, number of the risk factors amounts to dozens: presence of various microorganisms (human herpesvirus 1 and 6 (HHV-1 and 6) and Epstein-Barr virus (EBV), papovavirus, Semliki Forest virus, Visna virus, varicella zoster virus, Chlamydia pneumoniae, some mycoplasmas) is associated with development of MS and its animal analogues1-12. In this situation, it is clear that Koch's paradigm "one organism, one disease" cannot be applied to such inscrutable disease like MS. It is very speculative to connect any disease with any microorganism until the whole population will not be tested on the carriage of the same microbe. Any epidemiologist understands that it is a very difficult task. Unfortunately, the similar situation is observed in the MS genetics: at least 32 alleles of the major histocompatibility complex (MHC) are associated with development of MS13-14.
By our mind, the most logic are concepts that connect the MS pathogenesis with the dialogue disturbance between the nervous and immune systems, particularly due to imbalance of neurotransmitters. We try here to point out some issues connected to this problem.
The role of the neurotransmitter imbalance in MS and some other diseases
It is known by the moment that imbalance of neurotransmitters is an obligate condition in the MS patients as well as in people with other somatic and psychiatric disorders. For example, alterations in the serotonin content were described in details for MS patients both with relapsing-remitting, primary and secondary progressive forms15-18. Also, neurochemical disturbances were detected in patients with schizophrenia15, 19, cystic fibrosis20, pulmonary hipertension21, pancreatitis22, gallbladder muscle dysfunction23 and in many other cases. Moreover, some research groups showed evidently that reversibility of normal signalization within the CNS might result in normalization of functioning of various organs and systems15 and leading to improvement in people with neuroendocrine carcinoid syndrome24, bronchial asthma25, acute pancreatitis26 and during many other conditions.
Currently, neurological manifestations of MS are generally associated with the axon demyeliniation, which is considered also as an examination criterion27. However, clinical data suggest that a strong correlation between brain lesions and clinical presentation is absent. For example, as far back as last sixties researchers showed that the vision recovery after the optic neuritis episode cannot be explained by remyelination28. Moreover, it is known that sometimes people with intensive demyeliniation events may have no neurological deficit29. Also, brain lesions and spinal cord lesions may be observed accidentally in people long before development of the first clinical signs of MS30-33. The accumulated data suggest that the MS challenges cannot be considered as the direct manifestation of demyeliniation and that other possible factors, particularly imbalance of neurotransmitters, should be taken into account.
Over thirty years ago, the level of 5-hydroxyindoleacetic acid (5-HIAA) was shown to be decreased in the cerebral spinal fluid of patients with MS16, 17. Additionally, the level of the above-mentioned metabolite of serotonin was more decreased in the most severely disabled MS patients18. So, the presented data confirm a correlation between the level of cerebral serotonin and the disease course.
Since the beginning of sixties of the last century, intensive investigations of neurotransmitters and their metabolites are carried out in the Institute of Experimental Medicine (Caracas, Venezuela). Over 30000 healthy and diseased individuals were analyzed and some general conclusions were made. In particular, the investigators showed that patients with Th-1 immune profile (increased cellular immunity) display neurochemical features similar to those observed in major depression15. Namely, in patients with MS, Grave's ophthalmopathy, Crohn's disease, rheumatic arthritis, psoriasis and many others, a similar neurochemical disturbances are observed: increased norepinephrine to epinephrine ratio plus decreased level of tryptophan in the blood plasma. Alternatively, in Th-2 diseases (increased humoral immunity) - myasthenia, thrombocytopenic purpura, hemolytic anemia and others - the opposite neurochemical defects are detected (profile of maladaptation to stress)15. Numerous works by these authors have demonstrated that redressement of the observed neurotransmitter imbalance may result in improvement of patients. Moreover, one therapeutical scheme may work efficiently in patients with different disorders but incoming into one group (Th-1 or Th-2 diseases)15, 34-36.
It should be noted here that many studies have confirmed an entwinement between activity of immune and nervous systems37-38. Immunological changes observed in the MS patients are similar to those detected in the major depressed patients39. It is also known that depletion of the serotonin neurotransmission may be mediated by activation of indoleamine-2,3-dioxygenase (IDO) by inflammatory cytokines39. Some reseachers showed that the tryptophan derivates (3-hydroxy-kynurenine (3OH-KYN) and quinolinic acid (QUIN) arisen in the kynurenine cycle have neurotoxic effect40-41. Increased level of the above-mentioned metabolites is detected in neurodegenerative diseases and major depression40, 42. In turn, 3OH-KYN is able to induce the reactive oxygen species formation whose cytopathogenic effect is well documented43. Thus, the accumulated scientific data suggest neurochemical disturbances are obligate in any neurological disease and, as some investigators stated15, psychosomatic disorder. Therefore, prescription of some appropriate neurotrophic drugs able to restore neurotransmitter balance may be useful in many autoimmune diseases including MS.
Some steps in the MS neuroimmunomodulation
According to opinion of some authors15, enhancement of the central serotonin neurotransmission is an important step. The simplest way to increase serotonin neurotransmission is administration of the serotonin precursors like L-tryptophan and 5- hydroxytryptophan (5-HTP); the second might be synthesized in the human organism from the former. However, 5-HTP (a commercial product produced from the seeds of the African plant Griffonia simplicifolia) became the most popular and is used in clinical practice since the last seventies44. Efficiency of 5-HTP was confirmed in fibromyaligia, insomnia, chronic headaches and some other pathology45. This substance crosses the blood-brain barrier without difficulties, and increases significantly the serotonin synthesis in CNS46. Some vitamines and minerals may enhance serotonin synthesis in CNS. We have to note here that glucocorticoids frequently used for treatment of MS are able to induce IDO (an enzyme participating in the tryptophan catabolism) this fact is known since 1980s47.
In 1987, the selective serotonin reuptake inhibitors (SSRI) were introduced into clinical practice. Initially, this type of antidepressants was used entirely in psychiatric practice. However, during the last decade, some immunological activities of these drugs were described. At present, it is know that SSRI can cause significant decrease in synthesis of inflammatory cytokines and increase in the production of anti-inflammatory ones48-51 as well as mediate reduction of the interferon-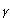 to interleukin 10 (INF-
to interleukin 10 (INF- 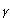 /IL-10) ratio whose importance for T-cell activation was shown previously52.
/IL-10) ratio whose importance for T-cell activation was shown previously52.
The presence of the increased levels of inflammatory cytokines in the MS patients was confirmed by many studies53-55. Therefore, we consider that application of SSRI and other serotonin promoting substances with the immune downregulation properties should be studied. Moreover, there were some positive results: administration of L-tryptophan to the MS patients resulted in improvement of autonomic, motor and sensory functions56. Expediency of the SSRI administration in the MS patients is under discussion now57. This type of antidepressant was shown to cause anti-inflammatory and anti-asthenia effects58-59 as well as have analgesic action60-61 that is very important for the MS sufferers.
CONCLUSION
Although close contact do exist between the CNS and immune system15, 62-63, it is impossible to conclude at present whether neurochemical disturbances are entirely related to MS pathogenesis or they are only consequences of autoimmune disease. Anyway, one thing is definitely clear that some neuropharmacological therapies aimed at correction of the neurotransmitter level and, as a result, modification of CNS and other nervous activities, may lead to improvements in patients with MS. Prescription of drugs with sympathomimetic actions (precursors of some neurotransmitters, some antidepressants, antagonist and agonists of 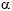 2- and ß2-adrenergic receptors, use of Bacillus Calmet-Guerin) may significantly reduce challenges of MS and cause other beneficial effects56, 59, 64-66. A vast experience obtained in the Institute of Experimental Medicine (Caracas, Venezuela) confirms the possibility to manage some autoimmune diseases without development of relapse during a long time15, 34-35. In addition, application of some physiotherapeutic procedures with the prior proserotonergic therapy may contribute to general improvement and long-term remission in the MS patients67-69. Taking into account low efficiency of currently used immunomodulators and their low safety profile70-71, prescription of unexpensive remedies with good tolerance and comparable efficiency seems rational decision. We hope that neurologists engaged in the MS study find the presented material valuable.
2- and ß2-adrenergic receptors, use of Bacillus Calmet-Guerin) may significantly reduce challenges of MS and cause other beneficial effects56, 59, 64-66. A vast experience obtained in the Institute of Experimental Medicine (Caracas, Venezuela) confirms the possibility to manage some autoimmune diseases without development of relapse during a long time15, 34-35. In addition, application of some physiotherapeutic procedures with the prior proserotonergic therapy may contribute to general improvement and long-term remission in the MS patients67-69. Taking into account low efficiency of currently used immunomodulators and their low safety profile70-71, prescription of unexpensive remedies with good tolerance and comparable efficiency seems rational decision. We hope that neurologists engaged in the MS study find the presented material valuable.
REFERENCES
1. Johnson RT. The virology of demyelinating diseases. Ann Neurol 1994; 36 (Suppl.): S54-60.
2. Soldan SS, Jacobson S. Role of viruses in etiology and pathogenesis of multiple sclerosis. Adv Virus Res 2001; 56: 517-55.
3. Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet 1985; 1: 1313-15.
4. Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 1993; 72: 551-60.
5. Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA 1995; 92: 7440-44.
6. Moore FG, Wolfson C. Human herpes virus 6 and multiple sclerosis. Acta Neurol Scand 2002; 106: 63-83.
7. Soldan SS, Leist TP, Juhng KN, Mc-Farland HF, Jacobson S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol 2000; 47: 306-13.
8. Wandinger KP, Jabs W, Siekhaus A, Bubel S, Trillenberg P.. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 2000; 55:178-84.
9. Martyn CN, Cruddas M, Compston DA. Symptomatic Epstein-Barr virus infection and multiple sclerosis. J Neurol 1993; Neurosurg Psychiatry 56: 167-68.
10. Sriram S, Mitchell W, Stratton C. Multiple sclerosis associated with Chlamydia pneumoniae infection of the CNS. Neurology 1998; 50: 571-72.
11. Sriram S, Stratton CW, Yao S, Tharp A, Ding L. Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis. Ann Neurol 1999; 46: 6-14.
12. Maida E. Immunological reactions against Mycoplasma pneumoniae in multiple sclerosis: preliminary findings. J Neurol 1983; 229: 103-11.
13. Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol 2004; 3: 104-10.
14. Haines JL, Bradford Y, Garcia ME, Reed AD, Neumeister E. Multiple susceptibility loci for multiple sclerosis. Hum Mol Genet 2002; 11: 2251-56.
15. Lechin F, van der Dijs B, Lechin ME. Neurocircuitry and Neuroautonomic Disorders: Reviews and Therapeutic Strategies. Basel, Karger, 2002.
16. Davidson D, Pullar IA, Mawdsley C, Kinloch N, Yates CM. Monoamine metabolites in cerebrospinal fluid in multiple sclerosis. J Neurol Neurosurg Psychiatry 1977; 40: 741-45.
17. Johansson B, Ross BE. 5-hydroxyindoleacetic acid and homovanillic acid in CSF of patients with neurological disease. Eur Neurol 1977, 11: 37-45.
18. Sonnien V, Riekkinen P, Rinne UK. Acid monoamine metabolites in cerebrospinal fluid in multiple sclerosis. Neurology 1973: 23: 760-63.
19. Lechin F, van der Dijs B. Noradrenergic hypothesis of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 2005; 29: 777-778.
20. Lechin F, van der Dijs B, Orozco B, Hernandez-Adrian G, Rodriguez S, Baez S. Similar autonomic nervous system disorders underlying cystic fibrosis and pancreatic cysts allowed common neuropharmacological therapy: Report of four cases. J Appl Res 2005; 5 :299-304.
21. Lechin F, van der Dijs B, Lechin AE. Pulmonary hipertension, left ventricular dysfunction and plasma serotonin. Br J Pharmacol 2002; 137: 937-938.
22. Lechin F, van der Dijs B, Lechin ME. Neuropharmacological factors, biliary motility and pancreatitis. J Pancreas 2002; 3: 152-154.
23. Lechin F, van der Dijs B, Orozco B. Gallbladder muscle dysfunction and neuroautonomic disorders. Gastroenterology 2002; 123: 1407-1408.
24. Lechin F, van der Dijs B, Orozco B, Rodriguez S, Baez S. Neuropharmacological therapy of the neuroendocrine carcinoid syndrome: Report of two cases. J Appl Res 2005; 5: 109-114.
25. Lechin F, van der Dijs B, Lechin AE. Treatment of bronchial asthma with tianeptine. Methods Find Exp Clin Pharmacol 2004, 26: 697-701.
26. Lechin F, van der Dijs B, Lechin M, Jara H, Lechin A, Cabrera A, Rada I, Orozco B, Jimenez V, Valderrama T. Dramatic improvement with clonidine of acute pancreatitis showing raised catecholamines and cortisol plasma levels: case report of five patients. J Med 1992; 23: 339-351.
27. Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol 1999; 12: 295-302.
28. Haymaker W. Bing's local diagnosis in neurological diseases. Saint Louis, The C.V. Mosby Company, 1969.
29. Sandyk R. Demyelination as an epiphenomenon in multiple sclerosis. Int J Neurosci 1993; 72: 141-148.
30. Russel DS. Trauma and multiple sclerosis. Lancet 1964; 1: 978.
31. Ghatak NR, Hirano A, Lijtmaer H, Zimmerman HM. Asymptomatic demyelinated plaque in the spinal cord. Arch Neurol 1974; 30: 484-86.
32. Phadke JG, Best PV. A typical and clinically silent multiple sclerosis: a report of 12 cases discovered unexpectedly at necropsy. J Neurol Neurosurg Psychiatry 1983; 46: 414-20.
33. Lynch SG, Rose JW, Smoker W, Petajan JH. MRI in familial multiple sclerosis. Neurology 1990; 40: 900-3.
34. Lechin F, van der Dijs B. Neuropharmacological therapy of carcinoid syndrome. Neuroendocrinology 2005; 81: 137-38.
35. Lechin F, van der Dijs B, Orozco B, Rodriguez S, Baez S. Neuropharmacological therapy of polycythemia vera: roles of circulating catecholamines and serotonin. Thromb Hemost 2005; 93: 175-77.
36. Lechin F, van der Dijs B, Orozco B, Jahn E, Rodriguez S, Baez S. Neuropharmacological treatment of refractory idiopathic thrombocytopenic purpura: roles of circulating catecholamines and serotonin. Thromb Haemost 2004; 91: 1254-56.
37. Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today 1994; 15: 504-11.
38. Savino W, Arzt E, Dardenne M. Immunoneuroendocrine connectivity: the paradigm of the thymus-hypothalamus-pituitary axis. Neuroimmunomodulation 1999; 6: 126-36.
39 Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 2005; 29: 201- 17.
40. Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci 2002; 71: 1837-48.
41. Wichers M, Maes M. The role of indoleamine 2,3 dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci 2004; 29: 11-17.
42. Mangoni A. The kynurenine shunt and depression. Adv Biochem Psychopharmacol 1974; 11: 293-98.
43. Hendriks JJA, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration Brain Res Rev 2005; 48: 185-95.
44. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypoth 2005; 65: 138-44.
45. Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev 1998; 3: 271-80.
46. Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: Supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther 2005; 109: 325-38.
47. Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J 1985; 229: 499-504.
48. Kubera M, Lin A, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol 2001; 21: 199-206.
49. Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol 2001; 16: 95-103.
50. Maes M, Song C, Lin A-H, Bonaccorso S, Kenis G. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology 1999; 20: 370-9.
51. Kubera M, Kenis G, Bosmans E, Scharpe S, Maes M. Effects of serotonin and serotonergic agonists and antagonists on the production of interferon-gamma and interleukin-10. Neuropsychopharmacology 2000; 23: 89-98.
52. Katsikis PD, Cohen SB, Londei M, Feldman M. Are CD4+ Th1 cells pro-inflammatory or anti-inflammatory? The ratio of IL-10 to INF-gamma or IL-2 determines their function. Int Immunol 1995; 7: 1287-94.
53. Carrieri PB, Provitera V, De Rosa T, Tartaglia G, Gorga F, Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical of IRS activation, such as T cell activation (increased activity. Immunopharmacol Immunotoxicol 1998; 20: 373-82.
54. Hautecoeur P, Forzy G, Gallois P, Demirbilek V, Feugas O. Variations of IL2, IL6, TNF alpha plasmatic levels in relapsing remitting multiple sclerosis. Acta Neurol Belg 1997; 97: 240-43.
55. Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol 2001; 11: 203-8.
56. Hyyppa MT, Jolma T, Riekkinen P, Rinne UK. Effects of L-tryptophan on central indoleamine metabolism and short-lasting neurologic disturbances in multiple sclerosis. J Neural Transm 1975; 37: 297-304.
57. Joffe RT. Depression and multiple sclerosis: a potential way to understand the biology of major depressive illness J Psychiatry Neurosci 2005; 30: 9-10.
58. Mohr DC, Goodkin DE, Islar J, Hauser BSL, Genain CP. Treatment of depression is associated with suppression of nonspecific and antigen-specific Th1 responses in multiple sclerosis. Arch Neurol 2001; 58: 1081-86.
59. Mohr DC, Hart SL, Golberg A. Effects of treatment for depression on fatigue in multiple sclerosis. Psychosom Med 2003; 65: 542-47.
60. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med 1997; 12: 384-89.
61. Duman EN, Kesim M, Kadioglu M, Yaris E, Kalyoncu NI, Erciyes N. Possible involvement of opioidergic and serotonergic mechanisms in antinociceptive effect of paroxetine in acute pain. J Pharmacol Sci 2004; 94: 161-65.
62. Sandyk R. Serotonergic neuronal atrophy with synaptic inactivation, not axonal degeneration, are the main hallmarks of multiple sclerosis. Int J Neurosci 1998; 95: 133-40.
63. Sandyk R. Tryptophan availability and the susceptibility to stress in multiple sclerosis: a hypothesis. Int J Neurosci 1996; 86: 47-53.
64. Guay AT, Spark RF, Jacobson J. Yohimbine treatment of organic erectile dysfunction in a dose-escalation trial. Int J Impot Res 2002; 14: 25-31.
65. Makhlouf K, Weiner HL, Khoury SJ. Potential of ß2-adrenoceptor agonist as add-on therapy fro multiple sclerosis: focus on salbutamol (albuterol). CNS drugs 2002; 16: 1-8.
66. Ristori G, Buzzi MG, Sabatini U. Use of Bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology 1999; 53: 1588-9.
67. Sandyk R. Progressive cognitive improvement in multiple sclerosis from treatment with electromagnetic fields. Int J Neurosci 1997; 89: 29-38.
68. Sandyk R. Successful treatment of multiple sclerosis with magnetic fields. Int J Neurosci 1992; 66: 237-50.
69. Sandyk R. Rapid normalization of visual evoked potential by picotesla range magnetic fields in chronic progressive multiple sclerosis. Int J Neurosci 1993; 77: 243-59.
70. Nousari HC, Asadi AK, Tausk FA. Subacute cutaneous lupus erythomatosus associated with interferon beta 1a. Lancet 1998; 352: 1825-1836.
71. Rotondi M, Mazziotti G, Biondi B. Long term treatment with interferon beta therapy for multiple sclerosis and occurrence of graves disease. J Endocrinol Invest 2000; 23: 321-324.
Markelov and Trushin correctly state that the etiology and pathogenesis is not yet well established in multiple sclerosis and make some suggestions about them. Although some are too general and based in
isolated data in diseases that have not much in common, they should be
considered for further investigation, as unconventional points of view
have often solved mysteries as is multiple sclerosis today.
The neurological and immunological systems share important characteristics such as their capability to learn from their experiences and to influence any organ of the economy. The neurotransmitters represent a sort of language that links both systems.
The authors proposed a very interesting hypothesis regarding the etiology of the multiple sclerosis which is attributed to a neurotransmitter imbalance: a reduction in the serotonin neurotransmission, and consequently the possibility to restore it pharmacologically.
This line of research could also explain the roots of many other entities as is the case of the autoimmune diseases as well as the psychosomatic disorders.
* Corresponding author:
Maxim V. Trushin
Received, March 25, 2006. Received reviewed: April 2, 2006
Comment of the reviewer José María Trejo Gabriel y Galán MD. PhD. Head of Neurology. Hospital General Yagüe. Burgos. Spain
Comment of the reviewer Carlos Musso, MD.Hospital Italiano. Buenos Aires. Argentina
As Dr Markelov and Dr Trushin pointed out in their article scarce works are devoted to multiple sclerosis (MS) pathogenesis. This situation is even reflected in the MS diagnosis criteria which are based on morphological aspects (demyeliniation areas without axonal damage and perivascular infiltration by inflammatory cells) instead of a physiopathological mechanism.
Kazan Institute of Biochemistry and Biophysics, P.O. Box 30, 420111, Kazan, Russia
Mail: mtrushin @ mail.ru
Published, April 3, 2006.
