Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
ADAPTATION TO UNFAVORABLE CONDITIONS OF GROWTH: PATHOGENICITY OF ACHOLEPLASMA LAIDLAWII PG8
Vladislav M. Chernov, Natalia E. Moukhametshina, Yurii V. Gogolev, Tatiana N. Nesterova,
Maxim V. Trushin and Olga A. Chernova
Kazan Institute of Biochemistry and Biophysics, Russian Academy of Sciences,
Kazan, Russia
mtrushin @ mail.ru
Rev Electron Biomed / Electron J Biomed 2006;3:11-15.
Comment of the reviewer Erhan Süleymanoglu, PhD. G.U.E.F., Department of Pharmaceutical Chemistry, Gazi University. Gazi Mahallesi, Ankara. Turkey
Comment of the reviewer Cord C. Uphoff, PhD. DSMZ - German Collection of Microorganisms and Cell Cultures. Department of Human and Animal Cell Lines. Braunschweig, Germany
As a result of cultivation of A. laidlawii PG8 cells on the deficient medium during 480 days, the mycoplasma culture adapted in vitro to unfavorable growth conditions was obtained. The culture consisted of cells with sizes less than 0.2 µm and features of A. laidlawii PG8 ultramicroforms, nanocells. A. laidlawii PG8 culture adapted in vitro to unfavorable growth conditions shows more evident phytopathogenicity than the unadapted one. Infecting plants V. minor L. by A. laidlawii PG8 culture adapted in vitro to UGC resulted in the appearance of chloroses in 75%, necrosis – 50%, leaves marcescence – 50% and abnormalities of bine development in 30% of plants through 12 days, while infecting plants by A. laidlawii PG8 culture unadapted to UGC led to respective signs in 40%, 25%, 25% and 0% of samples, respectively, through 30 days. The ability of A. laidlawii PG8 to form UMF resistant to stress factors in UGC with high phytopathogenic potential seems to demand a new approach to investigate the precise mechanisms of interacting the mycoplasma with host organisms.
KEY WORDS: Acholeplasma laidlawii PG8, phytopathogenicity, ultramicroforms, nanocells.
RESUMEN
Como resultado del cultivo de células de A. laidlawii PG8 en medio deficiente durante 480 días, fue obtenido un cultivo de mycoplasma adaptado in vitro a las condiciones desfavorables del crecimiento. El cultivo consistió en células con tamaño menor de 0.2 µm y características PG8 ultramicroformas de A. laidlawii nanocélulas. El cultivo de A. laidlawii PG8 adaptado in vitro a condiciones desfavorables del crecimiento muestra más evidente fitopatogenicidad que el inadaptado.
Plantas infectadas V. minor L. por el cultivo del A. laidlawii PG8 adaptado in vitro a UGC dio como resultado la aparición de clorosis en el 75%, necrosis en el 50%, marcescencia de las hojas en el 50% y anormalidades del desarrollo del bine en el 30% de plantas a los 12 días, mientras que las plantas infectadas por el cultivo del A. laidlawii PG8 inadaptado a UGC, condujo a dichos signos en el 40%, 25%, 25% y 0% de las muestras, respectivamente en 30 días. La capacidad del A. laidlawii PG8 a formar UMF resistente a los factores de estres en UGC con alto potencial fitopatogénico, parece exigir un nuevo planteamiento para investigar los mecanismos precisos de interacción entre el mycoplasma y el huesped.
PALABRAS CLAVE: Acholeplasma laidlawii PG8, fitopatogenicidad, ultramicroformas, nanocélulas.
INTRODUCTION
The limited biochemical possibilities of mycoplasmas, the smallest self-replicating prokaryotic organisms, are not an obstacle for these bacteria to spread in various biocenosia when persisting in higher eukaryotes and circulating in nature. In this connection, the molecular-genetic principles of the mycoplasma adaptation to unfavorable growth conditions of (UGC) seem to be of specific interest. However, only some initial steps were made in the investigation of the corresponding mechanisms 1,2. From the viewpoint of adaptive abilities, Acholeplasma laidlawii, a "ubiquitous" mycoplasma found in soil, compost, wastewaters, cellular tissues as well as in tissues of human beings, animals and plants, is a unique species 3. It was shown in our studies 1, 2 that the adaptive reactions of A. laidlawii PG8 to UGC were connected with transformation of vegetative forms of the mycoplasma cells into nanocells (ultramicroforms, UMF) resistant to biotic and abiotic stress factors. The phenomenon of nanotransformation was revealed in a number of bacteria, sometimes as a response reaction of microorganisms to UGC. In some bacteria, adaptation to UGC was followed by distortions of pathogenicity. We established previously 4, 5 that infecting plants by cells of A. laidlawii PG8 culture unadapted to UGC resulted in phytomycoplasmosis. The elucidating phytopathogenicity of A. laidlawii PG8 culture adapted to UGC was the aim of this study.
MATERIALS AND METHODS
A reference strain of Acholeplasma laidlawii PG8 obtained from the N.F. Gamalei Research Institute of Epidemiology and Microbiology was used in this study. Mycoplasma cells were cultivated for 2 days at 37 0C on the Edward’s medium to obtain unadapted to UGC culture. To obtain the mycoplasma culture adapted to UGC, glucose and yeast extract were eliminated from this impoverished medium; microorganisms were cultivated for 480 days with substrate deficiency 1. To detect titer of colony-forming units, the semi-fluid and solid-state variants of Edward’s medium with addition of 0.5% and 1.2% agarose were used. Two-dimensional protein electrophoresis, extraction, purification and electrophoretic separation as well as PCR analysis of DNA using specific AL16LF and A23LR primers for amplification of nucleotide sequences of A. laidlawii PG8 rRNA operons were performed as was described 2.
Phytopathogenicity of the adapted cultures and the unadapted one to UGC was investigated on enfleshed stalk of vinca Vinca minor L., a specific indicator of phytomycoplasmosis 6. Control and experimental groups consisted of 20 plants with the same size and age of rooting. Infecting plants in the experimental group was performed by Klement method 4 by injecting 1 µl culture adapted adapted in vitro to UGC A. laidlawii PG8 and unadapted one (stationary phase culture grown on the Edward’s full medium), respectively. In control group, plants were injected with 1 μl of sterile nutrient medium. Control and experimental plants were monitored during 6-8 weeks, a time necessary for manifestation of phytomycoplasmosis. The observed alterations were classified into types characteristic for mycoplasma plant infections 6: a delay of bine growth, chlorosis, necrosis, leaves marcescence and abnormalities in bine development.
The electron-microscopic analysis of the in vitro cultivation of mycoplasma cells was performed with the use of Hitachi-110 transmissible device (Hitachi, Japan). Ultra thin slices were obtained using LKB-III microtome (Sweden). The material under study was fixed with glutaric aldehyde (2.5%) prepared on the 0.1M phosphate buffer (pH 7.2) during 12 h. Then, the material was exsiccated using acetone and stored in 0,1% OsO4 with addition of 34 mg/ml of saccharose.
Statistical data processing was carried out using the standard mathematical methods (calculation of standard deviation, comparison of means by paired Student t-test) by means of Origin 6.0. A probability level of P<0.05 was considered significant to indicate differences between control and experimental groups 7.
RESULTS
As a result of cultivation of A. laidlawii PG8 cells on the deficient medium during 480 days, the mycoplasma culture adapted to UGC was obtained. The culture consisted of cells with sizes less than 0.2 μm and features of A. laidlawii PG8 UMF nanocells (Fig 1). On the solid medium, the UMF formed specific microcolonies in size from 50 to 300 μm rather than the typical mycoplasma ones, “fried eggs”. The UMF were able to revert into original vegetative forms of A. laidlawii PG8 cells when passaging them on the full Edward’s medium, showed evident viability and resistance to stress factors.
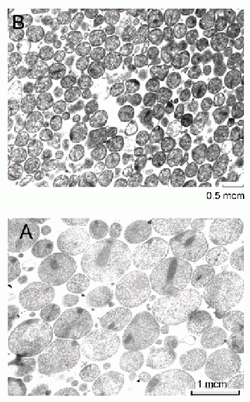
Figure 1. Transmission micrographics of A. laidlawii PG8 cells.
Cultures were grown on the Edward’s medium during 2 days (A)
and on the impoverished medium during 480 days (B).
Infecting plants V. minor L. by A. laidlawii PG8 culture adapted in vitro to UGC resulted in the appearance of chloroses in 75%, necrosis – 50%, leaves marcescence – 50% and abnormalities of bine development in 30% of plants through 12 days after injecting 105 CFU into plant tissues, while infecting plants by A. laidlawii PG8 culture unadapted to UGC led to respective signs in 40%, 25%, 25% and 0% of samples, respectively, through 30 days after injecting 107 CFU into plant tissues (Table 1).
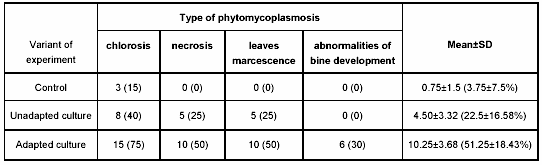
Table 1. A number of V. minor L. plants with various morphological deviations in control and experimental groups.
Note: absolute number of plants is indicated (percentage of the respective morphological deviations is shown in brackets).
The obtained data turned out to be evidence that adaptation in vitro of A. laidlawii PG8 culture to UGC was followed by a significant increase of its pathogenic potential.
DISCUSSION
It was previously found in our studies 1, 2 that adaptation of A. laidlawii PG8 culture to UGC was followed by nanotransformation – transformation of vegetative cell forms of the mycoplasma into UMF (nanocells) resistant to biotic and abiotic stress factors due to significant reorganization of genome expression. There were noticeable differences in morphologic, biochemical and molecular genetic properties between vegetative cell forms of the mycoplasma and its UMF.
It was shown a phenomenon of reverse attenuation of PCR signals for DNA of bacterial cells when cultivated at UGC [8]. The phenomenon of differential amplification of A. laidlawii PG8 rrnA and rrnB nucleotide sequences due to dissociation of the cell culture population was registered in our study (Fig 2). The data were proved to be useful to detect dissociation in cell population of the mycoplasma culture and to confirm transformation of the vegetative form of A. laidlawii PG8 cells into UMF in plant tissues.
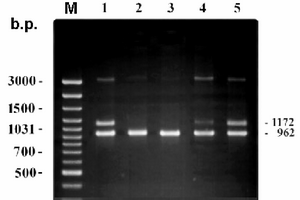
Figure 2. Differential amplification of rrnA and rrnB nucleotide sequences of A. laidlawii PG8 cells.
The lane M corresponds to marker for length of DNA fragments (b.p.). The lanes 1-5 contain PCR products of DNA extracted from the mycoplasma cells grown in full Edward's medium (1) and grown at unfavorable conditions (2,3 – unproliferated culture after 90 and 480 days of starvation, 4 – reverting culture – 1st passage after starvation during 480 days, 5 – active proliferating culture – 1st passage after starvation during 480 days).
Our previous experiments showed that infecting by A. laidlawii PG8 culture unadapted to UGC was followed by the appearance of ultrastructural, morphophysiological and biochemical deviations correlated with transformation of the vegetative cell forms of the mycoplasma into UMF 1, 2. In the present study we have mentioned that A. laidlawii PG8 culture adapted to UGC showed more expressive phytopathogenicity than the unadapted one. In plants infected by A. laidlawii PG8 unadapted to UGC culture the symptoms of the mycoplasma infection were detected at a later time, and were less evident in comparison with the adapted mycoplasma culture (Fig 3). Delayed manifestation of the mycoplasma infection symptoms in the plants infected by cells of A. laidlawii PG8 culture unadapted to UGC turned out to be related with the time-consuming processes of transformation of the vegetative cell forms into UMF. However, the molecular principles of more drastic phytopathogenicity of the mycoplasma UMF formed in vitro remain to clarify.
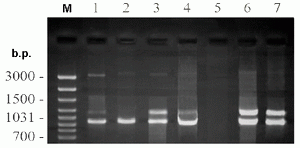
Figure 3. Detection of A. laidlawii PG8 by PCR with primers LA16LF and LA23LR in plants (V. minor L.). The lanes 1-4 contain PCR products of DNA extracted from plants infected with cells of adapted (1, 2) and unadapted (3, 4) culture of the mycoplasma through 4 days (1, 3) and 21 days (2, 4) after injecting. The lanes 5-7 correspond to negative (5) and positive (6, 7) controls – PCR products of DNA (230 ng) extracted from uninfected plants (5), mixed DNAs (230 ng / 10 ng) of uninfected plants and cells of unadapted culture of the mycoplasma (6) and DNA (10 ng) extracted from cells of unadapted culture of the mycoplasma (7). The lane M corresponds to marker for length of DNA fragments (b.p.).
Reorganization of genome expression in bacteria arising from UGC and determining the shift of bacterial metabolism was proved to result in distorting virulence of the microorganisms 9-11. It was found that the adaptive processes in some bacteria might be followed by the increase or decrease and even disappearance of pathogenicity. It was found for the first time in our study that adaptation of A. laidlawii PG8 culture to UGC in vitro was followed by an essential increase of phytopathogenic potential of the mycoplasma.
The ability of A. laidlawii PG8 to form UMF resistant to stress factors in UGC with high phytopathogenic potential seems to demand a new approach to investigate the precise mechanisms of interacting the mycoplasma with host organism.
ACKNOWLEDGEMENTS
The work was supported by the Russian Foundation for Basic Research (grant № 05-04-49435) and by the program of fundamental studies “Molecular and Cellular Biology” provided by the Russian Academy of Sciences as well as by Government contract №02.442.11.7283.
REFERENCES
- 1. Chernov VM, Gogolev YV, Mukhametshina NE, Abdrakhimov FA, Chernova OA. Mycoplasma adaptation to biogenic and abiogenic stressful factors; Acholeplasma laidlawii nannotransformation and minibodies. Dokl Biol Sci 2004; 396: 251-4.
2. Chernov VM, Gogolev YV, Mukhametshina NE, Abdrakhimov FA, Chernova OA. Adaptive reactions of mycoplasmas in vitro: "viable but unculturable forms" and nanocells of Acholeplasma laidlawii. Microbiologiya 2005: 74: 498-504.
3. Razin Sh, Herrmann R. Molecular biology and pathogenicity of mycoplasmas. NY, Plenum Publishers, 2002.
4. Chernov VM, Gogolev YV, Popova NV, Chernova OA. Infections of Pisum sativum of Acholeplasma laidlawii PG8 lead to change of morphological and physiological features of plants. Dokl Rus Acad Sci 1996; 348: 428-30.
5. Chernov VM, Gogolev YV, Popova NV, Chernova OA. Genetic variance of mycoplasmas (Acholeplasma laidlawii) during their interaction with eukaryotes (Pisum sativum). Dokl Rus Acad Sci 1999; 369: 275-77.
6. McCoy RE, deLeeuw GTN, Marwitz R, Chen TA, Cousin CJ, Sinha RC, Petzold H, Chiykowski LN, Coudwell A, Chang CJ, Dale JL, Golino D, Kirkpatrick B, Sugiura M, Whitcomb RF, Yang IL, Zhu BM, Seemuller E. Plant diseases associated with mycoplasma-like organisms. In: R. Whitcomb and JG Tully, eds. The mycoplasmas. Academic Press Inc, San Diego, 1989.
7. Sokal RR, Rohlf FJ. Biometry. The Principles and Practice of Statistics in Biological Research. San Francisco: Freeman, 1969.
8. Warner ZM, Oliver Z. Randomly amplified polymorphic DNA analysis of starved and viable but nonculturable Vibrio vulnigicus cells. Appl Environ Microbiol 1998: 64: 3025-28.
9. Baffone W, Citterio B, Vittoria E, Casaroli A, Campana R, Falzano L, Donelli G. Retention of virulence in viable but non culturable halophilic Vibrio spp. Int J Food Microbiol 2003, 89: 31-9.
10. Grey B, and Steck T. The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection. Appl Environ Microbiol 2001; 67: 3866-72.
11.Khmel' IA. Regulation of expression of bacterial genes in the absence of active cell growth. Genetika 2005; 41: 1183-1202.
The current work is devoted to clarifying phytopathogenicity of A. laidlawii PG8 culture following adaptation to unfavorable growth conditions. Having studied this trend of the adapted and unadapted cultures, employing stalk of vinca Vinca minor L., a specific indicator of phytomycoplasmosis, the authors classify the observed alterations into certain relevant types for mycoplasma plant infections. The electron microscopic analysis of in vitro cultivating mycoplasma cells and subsequent statistical evaluation of degrees of difference between control and experimental groups, shows that in vitro adaptation mechanisms of A. laidlawii PG8 culture to unfavorable growth conditions is followed by a significant increase of its pathogenic potential. Based on their previous studies on cellular nanotransformations of mycoplasmas, as well as on two-dimensional electrophoresis data, the authors emphasize the morphologic, biochemical and molecular genetic differences between vegetative form of cells and their nanocellular appearance. A possible explanation in terms of reorganization of mycoplasma genome expression during nanotransformation, resulting in distortion of virulence of microorganisms, is also mentioned.
The presented study shows that the in vitro adaptation of A. laidlawii PG8 culture to unfavorable growth conditions is followed by substantial increase of mycoplasma phytopathogenicity levels. Although, the suggested mechanisms remain to be clarified with further genetic analyses, the current work represents a good starting point for elucidating the molecular routes of mycoplasma interactions with host organisms and therefore deserves to be published.
The manuscript of Chernov et al. describes the effects of Acholeplasma laidlawii infections to Vinca minor plants after adapting the bacteria to unfavorable growth conditions. The study is based on a previously published paper, where the authors report the formation of nanocells (ultramicroforms) after growing the A. laidlawii cultures in medium without glucose and yeast extract. Infecting plants with nanoforms of A. laidlawii resulted in increased and more rapid phytopathogenicity compared to normal A. laidlawii cultures.
Comment of the reviewer Erhan Süleymanoglu, PhD. G.U.E.F., Department of Pharmaceutical Chemistry, Gazi University. Gazi Mahallesi, Ankara. Turkey
Comment of the reviewer Cord C. Uphoff, PhD. DSMZ - German Collection of Microorganisms and Cell Cultures. Department of Human and Animal Cell Lines. Braunschweig, Germany
Correspondence: Dr. Maxim V. Trushin,
Kazan Institute of Biochemistry and Biophysics,
Russian Academy of Sciences, Lobachevskiy str. 2/31, P.O. Box 30,
Kazan 420111, Russia;
E-mail: mtrushin @ mail.ru; Tel.:007-843-2319026.
Received, July 25, 2006. Received reviewed, November 6, 2006
Published, November 15, 2006
