Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HISTOLOGICAL STUDIES OF THE EFFECTS OF MONOSODIUM GLUTAMATE ON THE SMALL INTESTINE OF ADULT WISTAR RATS.
A.O.Eweka, F.A.E. Om'Iniabohs
Department of Anatomy. School of Basic Medical Sciences. College of Medical Sciences, University of Benin.
Benin City. Edo State. Nigeria.
andreweweka @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2007;2:14-18
Comment of the reviewer Dr. Luis A. Salazar-Olivo, MD. PhD. Instituto Potosino de Investigacion Cientifica y Tecnologica. Departamento de Biologia Molecular. Tangamanga. San Luis Potosi. Mexico.
Comment of the reviewer Roberto Cuan Ravinal MD. PhD. Departamento de Patologia. Faculdade de Medicina de Ribeirão Preto, FMRP-USP. Ribeirão Preto; Centro Universitário de Araraquara, UNIARA, Araraquara. Brasil
ABSTRACT:
The effects of monosodium glutamate (MSG) used as food additive on the small intestine of adult wistar rat was investigated. Both adult male and female Wistar rats (n=24) of average weight of 185g were randomly assigned into two treatments (n=16) and control (n=8) groups. The rats in the treatment groups received 3g and 6g of MSG thoroughly mixed with grower's mash daily for fourteen days. The control rats received equal amounts of grower's mash without MSG added daily. The grower's mash was obtained from Edo Feeds and Flour Mill Ltd, Ewu, Edo State and the rats were given water ad libitum. The rats were sacrificed on day fifteen of the experiment. The small intestine was carefully dissected out and quickly fixed in 10% formal saline for routine histological procedures.
The histological findings in the treated groups showed evidence of increased basophilia and cellular hypertrophy in animals given 3g of MSG, while degenerative and atrophic changes in the group with 6g of MSG was more pronounced. These findings indicate that Monosodium glutamate may have some deleterious effects on the microanatomy of the small intestine of adult Wistar rats at higher doses. It is recommended that further studies aimed at corroborating these findings be carried out.
Key words: MMonosodium glutamate. Histological effect. Small intestine. Wistar rats.
INTRODUCTION
Monosodium glutamate (MSG) otherwise known as AJI-NO-MOTO or white maggi is the sodium salt of glutamic acid. Glutamate is one of the most common amino acids found in nature and is the main component of many proteins and peptides of most tissues. Monosodium glutamate contains 78% of glutamic acid, 22% of sodium and water1. Glutamate is also produced in the body and plays an essential role in human metabolism. It is a major component of many protein-rich food products such as meat, fish, milk and some vegetables2.
In 1908, Professor Kikunae Ikedia of the University of Tokyo Japan, working on the seasoning abilities of some seaweed isolated MSG. A year later the patent was bought and the new seasoning was christened Aji-no-moto3. Today, MSG is produced in many countries around the world through a fermentation process of molasses from sugar cane or sugar beets, as well as starch and corn sugar2,3. In this fermentation process, bacteria, which may be genetically modified, are grown aerobically in a liquid nutrient medium. These bacteria have the ability to synthesize glutamic acid outside of their cell membranes and excrete it into the medium to accumulate there4. When MSG is added to food, it provides a flavoring function similar to the naturally occurring free glutamate: which differ from the four classic tastes of sweet, sour, salty and bitter.
The toxic effect of MSG was further corroborated by the work done on the testis, causing significant oligozoospermia and increase abnormal sperm morphology in a dose-dependent fashion in male wistar rats5. It has also being established that MSG may be implicated in cases of male infertility as it causes testicular hemorrhage, degeneration and alteration of sperm cell population and morphology6.
In Nigeria, most communities and individuals often use Monosodium glutamate as a bleaching agent for the removal of stains from clothes. There is a growing apprehension that its excellent bleaching properties could be harmful or injurious to the intestinal mucosa, or worse still inducing terminal diseases in consumers when ingested as a flavor enhancer in food. Despite evidence of negative consumer response to MSG, reputable international organizations and nutritionist have continued to endorse Monosodium glutamate, and reiterate that Monosodium glutamate has no adverse reactions in humans5. The Food and Drug Administration (FDA) of the United States reports that Monosodium glutamate is safe and that it should be maintained on the "Generally Recognized as Safe" (GRAS)-list of foods; being listed on food labels as a "Flavoring" or "hydrolyzed vegetable protein". MSG is thus reportedly permitted as a safe food additive that needs no specified average, daily intake or an upper limit intake requirement. The Directorate and Regulatory Affairs of Food and Drug Administration and Control (FDA & C) in Nigeria, now NAFDAC has also expressed the view that MSG is not injurious to health7.
Through its stimulation of the orosensory receptors and by improving the palatability of meals, MSG influences the appetite positively, and induces weight gain8. Despite its taste stimulation and improved appetite enhancement, reports indicate that MSG is toxic to human and experimental animals9.
In 1968, the first published report of an adverse reaction to Monosodium glutamate appeared in the New England Journal of Medicine where it was reported that Monosodium glutamate was neurotoxic; killing brain cells, causing retinal degeneration, endocrine disorder and also associated with a number of pathological conditions such as addition, stroke, epilepsy, brain trauma, neuropathic pain, schizophrenia, anxiety, depression, degenerative disorders such as Parkinson's disease, Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis1, 10. MSG causes increase in alkaline phosphatase activity in the small intestine10,
The small intestine functions in the digestion and absorption of food materials in the body. It also prevents duodenal ulceration due to the presence of the Brunner's gland. The small intestine consists of the duodenum, jejunum and ileum. Since MSG causes increase in alkaline phosphatase activity in the small intestine, it is relevant to investigate some of its histological effects on the small intestine. It is probable that the adverse effects of MSG may affect the normal physiological action of the small intestine and hence this investigation.
MATERIALS AND METHODS
ANIMALS:
Thirty, (24) adult wistar rats of both sexes with average weight of 185g were randomly assigned into three groups A, B and C of (n-8) in each group. Groups A and B of (n-16) serves as treatments groups while Group C (n-8) is the control. The rats were obtained and maintained in the Animal Holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Nigeria. They were fed with grower's marsh obtained from Edo feed and flour mill limited, Ewu, Edo state) and given water liberally. The rats gained maximum acclimatization before actual commencement of the experiment. The Monosodium glutamate (3g/ sachet containing 99+% of MSG) was obtained from Kersmond grocery stores, Uselu, Benin city.
MONOSODIUM GLUTAMATE ADMINISTRATION:
The rats in the treatment groups (A and B) were given 3g and 6g of MSG
Thoroughly mixed with the grower's marsh, respectively on a daily basis. The control group (C) received equal amount of feeds (Grower's march) without MSG added for fourteen days. The rats were sacrificed on the fifteenth day of the experiment. The abdominal region was opened to expose the abdominal visceral.
The small intestine was quickly dissected and fixed in 10% formal saline for routine histological techniques. The 3g and 6g MSG doses were chosen and extrapolated in this experiment based on the indiscriminate use here in Nigeria due to its palatability. The two doses were thoroughly mixed with fixed amount of feeds (550g) in each group daily.
HISTOLOGICAL STUDY:
The tissue were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7 microns thick were obtained using a rotatory microtome. The deparaffused sections were stained routinely with haematoxyline and eosin.
Photomicrographs of the desired results were obtained using digital research photographic microscope.
RESULTS
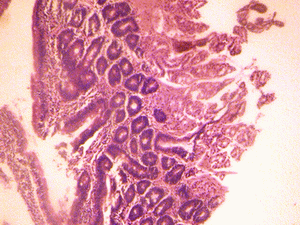
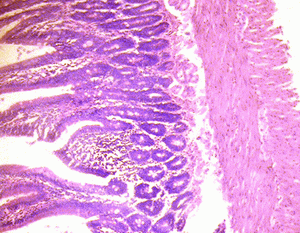
The small intestine of the control group showed normal histological features of the duodenum and jejunum with distinct long villi, tall columnar epithelium, and few crypts of Lieberkuhn. There were numerous Brunner's glands and few Payers' patches in the duodenum and jejunum respectively (Figure 1A & 2A).
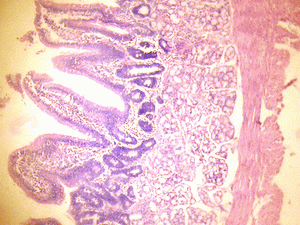 Figure 1A: Control section of duodenum (Mag. x400). |
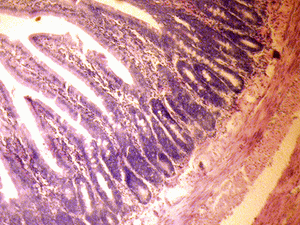 Figure 2A: Control section of the jejunum (Mag. x400). |
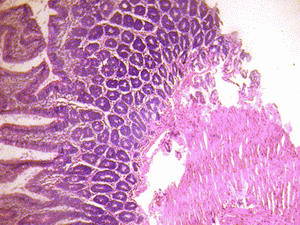
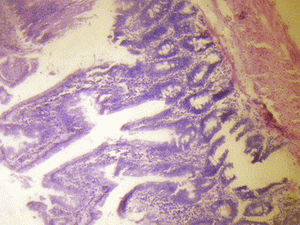
The small intestine of the treated groups showed increased basophilia in the nucleus with the animals in the group receiving 6g more marked, and some varying cellular hypertrophy. There were distortions in the epithelia of the small intestine. There were no obvious histological changes in Brunner's glands of the duodenum and the Payers patches of the jejunum in the group that received 3g of MSG (figure 1B & 2B). However degenerative and atrophic changes were observed in the sections of the duodenum and jejunum in the group that received 6g of MSG (figure1C& 2C).
DISCUSSION
The results of the haematoxylin and eosin staining (H & E) reactions showed intensed staining of the nuclei, which is an evidence of increased basophilia, increased cellular hypertrophy and cellular proliferation in the small intestine of the treatment groups. The increased basophilia is an indication that there is an increase in mucous protein synthesis by the rough endoplasmic reticular consequent upon increase intake of food due to the palatability of MSG10. This corroborates the fact that MSG causes an increase in appetite and thereby leading to increase in weight and obesity10. The increase in cellular hypertrophy of the treatment group as reported in this study may have been as a result of cellular proliferation caused by the improved intake of food which MSG influences8. There were degenerative and atrophic changes observed in the small intestine, especially in the group treated with higher dose (0.08g/kg) of MSG.
It may be inferred from the present results that higher dose and prolonged administration of MSG resulted in degenerative and atrophic changes observed in the Brunner's glands of the duodenal portion of the small intestine. The actual mechanism by which MSG induced cellular degeneration observed in this experiment needs further investigation.
Degenerative changes have been reported to result in cell death, which is of two types, namely apoptotic and necrotic cell death. These two types differ morphologically and biochemically11. Pathological or accidental cell death is regarded as necrotic and could result from extrinsic insults to the cell such as osmotic, thermal, toxic and traumatic effects12. In this experiment MSG could have acted as toxins to the cell of the small intestine. The process of cellular necrosis involves disruption of membrane's structural and functional integrity which was also a landmark of this experiment. In cellular necrosis, the rate of progression depends on the severity of the environmental insults.
The greater the severity of insult, the more rapid the progression of neuronal injury13 .The principle holds true for toxicological insults to the brain and other organs14. It may be inferred from the present results that prolonged intake of MSG resulted in increase toxic effects on the small intestine with that of higher dose more marked.
CONCLUSION AND RECOMMENDATION
The results obtained in this study following the administration of 0.04g/kg and 0.08g/kg per day of MSG to adult wistar rats causes increased basophilia and cellular hypertrophy. The Brunner's glands of the duodenum whose rats received 0.08g/kg of MSG were observed to have undergone some degenerative and atrophic changes. It is recommended that further studies be carried out to corroborate these findings.
REFERENCES
-
1.- Adrienne Samuels: The Toxicity/Safety of MSG; A study in suppression of information. Accountability in Research. 1999;6:259-310.
2.- IFIC Review of Monosodium glutamate: Examining the myths (1994).
3.- K-othmer Encyclopedia of Chemical Technology. Fourth edition (Wiley 1992) Pp 571-579.
4.- Leung A, Foster S. Encyclopaedia of common Natural ingredient used in foods, drugs and cosmetics. New York (Wiley1996) pp373-375
5.- Onakewhor JUE, Oforofuo IAO, Singh SP: Chronic administration of Monosodium glutamate Induces Oligozoospermia and glycogen accumulation in Wister rat testes. Africa J. Reprod Health 1998;2:190-197
6.- Oforofuo IAO, Onakewhor JUE, Idaewor PE: The effect of Chronic Admin Of MSG on the histology of the Adult wister rat testes: Bioscience Research Communications Vol 9, No. 2, June 30, 1997
7.- Okwuraiwe PE: The role of food and Drug Administration and control FDA&C) in ensuring the safety of food and food ingredients: A symposium held at Sheraton Hotel, Lagos. 1st Sept.1992: 6-15.
8.- Rogers PP, Blundell JE: Umani and appetite: Effects of Monosodium glutamate on hunger and food intake in human subjects. Phsiol. Behav. 1990:486:801-804.
9.- Belluardo M, Mudo G and Bindoni M: Effect of early destruction of the mouse arcuate nucleus by MSG on age dependent natural killer activity. Brain Res. 1990;534:225-333
10.- Mozes S, Sefcikova Z: Obesity and changes of alkaline phosphatase activity in the small intestine of 40 and 80-day old rats subjected to early postnatal overfeeding of monosodium glutamate.- Physiol Res. 2004;53:177-86.
11.- Wyllie AH: Glucocorticoid-induced thymocyte apoptosis is associated and endogenous endonuclease activation. Nature, London 1980;284:555-556.
12.- Farber J L Chein K R and Mittnacht S The pathogenesis of irreversible cell injury in ischemia. American Journal of Pathology 1981;102:271-281.
13.- Ito U, Sparts M, Walker Jt and Warzo: Experimental Cerebral Ischemia in Magolian Gerbils: Light microscope observations. Acta Neuropatholog: USA 1975;32:209-223.
14.- Martins LJ, Deobler JA, Shih T, Anthony A: Cytophotometric analysis of thalamic neuronal RNA in some intoxicated rats. Life Sci. 1984;35:1593-1600
Received October 4, 2006. Received reviewed May 29, 2007
Published June 16, 2007