Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HISTOLOGICAL STUDIES OF THE EFFECTS OF MONOSODIUM GLUTAMATE ON THE MEDIAL GENICULATE BODY OF ADULT WISTAR RATS.
A.O.Eweka, J.O. Adjene
Department of Anatomy. School of Basic Medical Sciences.
College of Medical Sciences, University of Benin.
Benin City. Edo State. Nigeria.
andreweweka @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2007;2:9-13
Comment of the reviewer Dr. José María Trejo Gabriel y Galán, MD. PhD. Department of Neurology. Hospital General Yagüe. Complejo Asistencial de Burgos. España
Comment of the reviewer Maxim V. Trushin PhD. Laboratory of Pathogenesis. Kazan Institute of Biochemistry and Biophysics. Russian Academy of Sciences. Kazan Russia
ABSTRACT:
Histological effects of Monosodium glutamate (MSG) commonly used as food additive on the medial geniculate body (MGB) of adult wistar rats were carefully studied. The rats of both sexes (n=24), average weight of 185g were randomly assigned into two treatments (n=16) and control (n=8) groups.
The rats in the treatment groups received 3g and 6g of MSG thoroughly mixed with their feeds for fourteen days, while the control rats received equal amounts of feeds without MSG added. The rats were fed with grower's mash purchased from Edo Feeds and Flour Mill Ltd, Ewu, Edo State and were given water liberally. The rats were sacrificed on day fifteen of the experiment. The medial geniculate body was carefully dissected out and quickly fixed in 10% formal saline for routine histological study after H&E method.
The histological findings after H&E methods indicated that the treated sections of the medial geniculate body showed some cellular degenerative changes, autophagic vacuoles with some vacuolations appearing in the stroma, and some degree of neuronal hypertrophy when compared to the control sections. These findings indicate that MSG consumption may have a deleterious effect on the neurons of the medial geniculate body (MGB). MSG may probably have adverse effects on the auditory sensibilities by its deleterious effects on the nerve cells of the MGB of adult wistar rats. It is recommended that further studies aimed at corroborating these observations be carried out.
Key words: Monosodium glutamate, Histological effect, medial geniculate body and wistar rats.
INTRODUCTION
Most food additives act either as preservatives or enhancer of palatability. One of such food additive is monosodium glutamate (MSG) and it is sold in most open markets and stores in Nigeria as "Ajinomoto" marketed by West African Seasoning Company Limited; as "Vedan" or "White Maggi" marketed by Mac and Mei (Nig) Limited. Various environmental chemicals, industrial pollutants and food additives have been implicated as causing harmful effects1.
The safety of MSG's usage has generated much controversy locally and globally2. In Nigeria, most communities and individuals often use MSG as a bleaching agent for the removal of stains from clothes. There is a growing apprehension that its excellent bleaching properties could be harmful or injurious to the stomach mucosa, or worse still inducing terminal diseases in consumers when ingested as a flavor enhancer in food. Despite evidence of negative consumer response to MSG, reputable international organizations and nutritionist have continued to endorse MSG, reiterating that it has no adverse reactions in humans. Notably of such is the Directorate and Regulatory Affairs of Food and Drug Administration and Control (FDA&C) in Nigeria, now NAFDAC has also expressed the view that MSG is not injurious to health3.
MSG improves the palatability of meals and thus influences the appetite centre positively with it resultant increase in body weight4. Though MSG improves taste stimulation and enhances appetite, reports indicate that it is toxic to human and experimental animals5. MSG has a toxic effect on the testis by causing a significant oligozoospermia and increase abnormal sperm morphology in a dose-dependent fashion in male wistar rats6. It has been implicated in male infertility by causing testicular hemorrhage, degeneration and alteration of sperm cell population and morphology7. It has been reported that MSG has neurotoxic effects resulting in brain cell damage, retinal degeneration, endocrine disorder and some pathological conditions such as addition, stroke, epilepsy, brain trauma, neuropathic pain, schizophrenia, anxiety, depression, Parkinson's disease, Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis8, 9. It cannot be stated that MSG is the cause of such varied conditions as epilepsy and Alzheimer's disease, although there may be concerns of its involvement in its etiology.
The medial geniculate body is the target of ascending projections from the inferior colliculus and descending input from the auditory cortex; this is the obligatory synaptic target in the thalamus for hearing. It contains interleaved and overlapping tonotopic and aural bands, the most beautiful structure in the brain10. The cerebral cortex strongly affects the medial geniculate body through descending projections which are thought to consist primarily of small areas with slow conduction velocities11. Although the MGB receives projections from the auditory cortex its connections are mainly towards the auditory cortex. Since it has been reported that MSG has a neurotoxic effect, it is worthwhile to investigate its effects on the medial geniculate body. This work is carried out to investigate some probable histological effects of MSG on the MGB, being an obligatory synaptic sub cortical target structure for hearing in adult wistar rats.
MATERIALS AND METHODS
ANIMALS:
Twenty four (24) adult wistar rats of both sexes with average weight of 185g were randomly assigned into three groups A, B and C of (n=8) in each group. Groups A and B of (n=16) serves as treatments groups while Group C (n=8) is the control. The rats were obtained and maintained in the Animal Holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Nigeria. They were fed with grower's marsh obtained from Edo feed and flour mill limited, Ewu, Edo state) and given water liberally. The rats gained maximum acclimatization before actual commencement of the experiment. The Monosodium glutamate (3g/sachet containing 99+% of MSG) was obtained from Kersmond grocery stores, Uselu, Benin City.
MONOSODIUM GLUTAMATE ADMINISTRATION:
The rats in the treatment groups (A and B) were given 3g and 6g of MSG, thoroughly mixed with the grower's marsh, respectively on a daily basis. The control © group received equal amount of feeds (Grower's march) without MSG added for fourteen days. The rats were sacrificed on the fifteenth day of the experiment. The skulls were opened using bone forceps to expose the brain of the rats. The medial geniculate body was quickly dissected out, weighed and fixed in10% formal saline for routine histological techniques. The 3g and 6g MSG doses were chosen and extrapolated in this experiment based on the indiscriminate use here in Nigeria due to its palatability. The two doses were thoroughly mixed with fixed amount of feeds (550g) in each group daily.
HISTOLOGICAL STUDY:
The tissue were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7 microns thick were obtained using a rotatory microtome. The deparaffused sections were stained routinely with haematoxyline and eosin. Photomicrographs of the desired sections were made for further observations.
RESULTS
The control sections of the medial geniculate body showed normal histological features with the neurons appearing distinct and the glial cells normal without vacuolations in the stroma (Figure 1).

Figure 1: Control section of the medial geniculate body. Mag. x 400.
The treatment sections of the medial geniculate body showed some cellular degenerative changes; vacuolations appearing in the stroma with some autophagic vacuoles. There is also some degree of neuronal hypertrophy which is more marked in the treatment sections receiving 6g of MSG (Figures 2, 3).
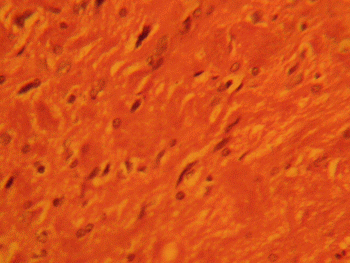
Figure 2: Treatment section of the medial geniculate body
(3g MSG) Mag. x 400.
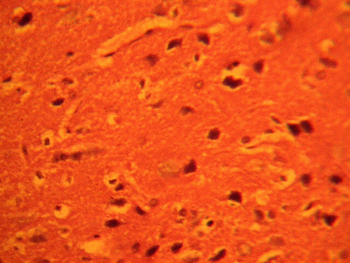
Figure 3: Treatment section of the medial geniculate body
(6g MSG) Mag. x 400
DISCUSSION
The results of the haematoxylin and eosin staining (H&E) reactions revealed that with increasing dose of monosodium glutamate consumption caused some cellular degenerative changes with vacuolation appearing in the stroma of the treatment group compared to the control sections of the medial geniculate body.
Neuronal degeneration has been reported to result in cell death, which is of two types, namely apoptotic and necrotic cell death. These two types differ morphologically and biochemically12. Pathological or accidental cell death is regarded as necrotic and could result from extrinsic insults to the cell such as osmotic, thermal, toxic and traumatic effects13. It was reported that cell death in response to neurotoxins might trigger an apoptotic death pathway within brain cells14. Cell death in response to neurotoxins occurs as a controlled event involving a genetic programme in which caspase enzymes are activated14.
The vacuolations observed in the stroma of the treated medial geniculate body may be due to MSG interference. The toxic effects of MSG on the weight of the medial geniculate body observed in this experiment may underline the possible neurological symptoms already reported.
MSG administration resulted in excitotoxic neuronal degeneration of the cerebella development in chick15. It has been reported that MSG produces neuronal degeneration in several brain regions when administered in neonatal rats16. The vacuolations observed in this experiment is associated with increase in the weight of the treated groups compared to the control sections o the medial geniculate body. As brain swells as seen in this study the activities of cellular transporters is approximately modified by up or down regulations as earlier reported in the case of hyponatremia or hypernatremia17.Ischaemic or pharmacologic disruption of cellular transporters can cause swelling of parenchyma of the medial geniculate body. The disruption of MSG is a cardinal feature of the results of this experiment. Though there are many different causes of cell swelling including drug poisoning, water intoxication, hypoxia from asphyxia and acute hypoatremia17.The cytotoxic oedema associated with this experiment usually involves intracellular swellings of glial, endothelia and neurons17. Brain swellings attendant to severe cytotoxic oedema will lead to marked reduction in the size of the ventricular system and basal cisterns17. MSG may have acted as toxins to the cells of the medial geniculate body, affecting their cellular integrity and causing defect in membrane permeability and cell volume homeostasis.
The neuronal hypertrophy observed in this experiment may have been caused by the cytotoxic effect of MSG on the medial geniculate body. This obviously will affect the normal propagation of impulses in this intracranial auditory relay centre. Normal conduction of impulse involves the propagation of action potential along nerve fibers in the form of wave of activity that constitutes the nerve impulse18. The neuronal hypertrophy which is associated with some pyknotic neurons in the stroma of the medial geniculate body is also in consonance with the work reported by Adjene and Caxton Martins19.
CONCLUSION AND RECOMMENDATION
The results obtained in this study revealed that monosodium glutamate consumption could affect the histology of the medial geniculate body. The nerve cells of the treated sections of the medial geniculate body showed some cellular degenerative changes, autophagic vacuoles and neuronal hypertrophy. With this result it is probable that the functions of the medial geniculate body as an intracranial auditory centre may be adversely affected. It is recommended that further studies be carried out to corroborate these findings.
REFERENCES
1.- Moore KL: congenital malformations due to environmental; Developing Humans. W.B. Saunders co. Ltd. Philadelphia.2003; 2nd ed.pp.173-183.
2. Biodun D, Biodun A: A spice or poison? Is monosodium glutamate safe for human consumption? National concord 1993; 4th Jan. p5.
3.- Okwuraiwe PE: The role of food and Drug Administration and control (FDA&C) in ensuring the safety of food and food Ingredients: A symposium held at Sheraton Hotel, Lagos. 1st Sept.1992: 6-15.
4.- Rogers PP, Blundell JE: Umani and appetite: Effects of monosodium glutamate on hunger and food intake in human subjects. Phsiol. Behav. 1990: 486:801-4.
5.- Belluardo M, Mudo G and Bindoni M: Effect of early Destruction of the mouse arcuate nucleus by MSG on age Dependent natural killer activity: Brain Res.1990, 534:225-333
6.- Onakewhor JUE, Oforofuo IAO, Singh SP: Chronic Administration of Monosodium glutamate Induces Oligozoospermia and glycogen Accumulation in Wister rat testes. Africa J Reprod Health 1998; 2: 190-197.
7.- Oforofuo IAO, Onakewhor JUE, Idaewor PE: The effect of chronic admin of MSG on the histology of the Adult wister rat Testes: Bioscience Research Communications. 1997 Vol. 9, No. 2
8.- Adrienne Samuels: The Toxicity/Safety of MSG; A study in suppression of information. Accountability in Research 1999: 6(4): 259-310.
9.- Mozes S, Sefcikova Z: Obesity and changes of alkaline phosphatase activity in the small intestine of 40 and 80-day old rats subjected to early postnatal overfeeding of monosodium glutamate. Physiol Res. 2004. 53 (2), 177-86
10.- Fall: Mammalian Neuroanatomy MCB 163: 1999
11.- Winer JA, Saint Marie RL, Larue DT, Oliver DL: The cerebral Cortex strongly affects the medial geniculate body through descending projections: Proc.Nati.Acad.Sci. USA. 1996; 93:8005-8010
12.- Wyllie AH: Glucocorticoid-induced thymocyte apoptosis is Associated and endogenous endonuclease activation. Nature: London 1980; 284:555-556.
13.- Farber JL Chein KR, Mittnacht S: The pathogenesis of irreversible cell injury in ischemia; American Journal of Pathology 1981; 102:271-281
14 Waters CM, Wakinshaw G, Moser B, Mitchell IJ: Death of neurons in the neonatal rodent globus pallidus occurs as a mechanism of apoptosis. Neuroscience: 1994; 63: 881-894.
15.- Espinar A, Garcia-Oliver A et al : Neuroprotection by melatonin from glutamate-induced exocitoxicity during development of the cerebellum in the chick embryo: J. pineal Res. 2000; 2: 818.
16.- Urena-Guerrero ME, Lopez-Perez SJ, Beas Zaratel B : Neonatal Monosodium glutamate treatment modified glutamic acid decarboxylase activity during rat brain postnatal development: Neurochem. Int. 2003; 42(4): 269-276.
17.- Johnson CE : Effects of fluid imbalances: Neurosciencee in Medicine: P. Michael conn JB Lippincott Company. 1995; pp. 187-189.
18.- Gispen WH : Molecular and functional neurobiology: Elsevier Publishing Company, USA. 1976 102-104.
19.- Adjene JO, Caxton-Martins AE : Some histological effect of chronic administration of Chloroquine on the medial geniculate body of adult wistar rat Afri. J. Med. Sci. (2006): 35: 131-135.
