Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HISTOLOGICAL STUDIES OF THE EFFECTS OF ORAL ADMINISTRATION OF ARTESUNATE
ON THE MEDIAL GENICULATE BODY OF ADULT WISTAR RATS.
A.O.Eweka, J.O. Adjene
Department of Anatomy. School of Basic Medical Sciences. College of Medical Sciences, University of Benin.
Benin City. Edo State. Nigeria.
andreweweka @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2008;1:20-26
Comment of the reviewer Prof Pilar Muñiz Rodríguez, PhD. Titular del Área de Bioquímica y Biología Molecular de la Facultad de Ciencias de la Universidad de Burgos. España
Comment of the reviewer Erhan Süleymanoglu. PhD. G.U.E.F., Department of Pharmaceutical Chemistry, Gazi University. Gazi Mahallesi, Ankara. Turkey
ABSTRACT:
The histological effect of oral administration of artesunate, commonly used for the treatment of Malaria on the medial geniculate body (MGB) of adult wistar rat was carefully studied. The rats of both sexes (n=24), average weight of 210g were randomly assigned into three treatment (n=18) and control (n6) groups.
The rats in the treatment group 'A' received 4mg/kg body weight of artesunate base dissolved in distilled water for 3 days. The animals in groups 'B' and 'C' received 4mg/kg body weight of artesunate dissolved in distilled water for the first day and thereafter received 2mg/kg body weight daily for six and thirteen day respectively. The control group D, received equal volume of distilled water daily using the Orogastric tube. The rats were fed with grower's mash obtained from Edo Feeds and Flour Mill Ltd, Ewu, Edo State, Nigeria and were given water liberally. The rats were sacrificed on day fourth, eight and fifteenth of the experiment. The medial geniculate body was carefully dissected out and quickly fixed in 10% formal saline for histological studies.
The histological findings after H&E method indicated that the treated section of the medial geniculate body showed some decreased cellular population, degenerative changes, cellular hypertrophy, with some vacuolations appearing in the stroma.
Varying dosage and long administration of artesunate may have some deleterious effects on the neurons of the Medial geniculate body and this may probably have some adverse effects on auditory sensibilities by its deleterious effects on the cells of the medial geniculate body of adult wistar rats. It is therefore recommended that further studies aimed at corroborating these observations be carried out.
Key words: Artesunate, medial geniculate body, decrease cellular population, vacuolation.
RESUMEN: ESTUDIO HISTOLÓGICO DE LOS EFECTOS DE LA ADMINISTRACIÓN ORAL DE ARTESUNATO EN CUERPO GENICULADO MEDIO DE RATAS WISTAR ADULTAS
El efecto histológico de la administración oral de artesunate, comúnmente utilizado para el tratamiento de la malaria en el cuerpo geniculado medio (MGB) de ratas wistar adultas, fue cuidadosamente estudiado. Ratas de ambos sexos (n = 24), con peso promedio de 210 g fueron distribuidas de forma aleatoria en tres grupos de tratamiento (n = 18) y control (n = 6).
Las ratas del grupo de tratamiento "A" recibieron 4mg/kg peso corporal de artesunate base, disuelto en agua destilada, durante 3 días. Los animales en los grupos "B" y "C" recibieron 4mg/kg peso corporal de artesunate disuelto en agua destilada el primer día y posteriormente recibieron 2mg/kg peso corporal al día durante seis y trece días respectivamente. El grupo control D, recibió igual volumen de agua destilada al día, utilizando sonda nasográstrica. Las ratas fueron alimentadas con pienso de Edo Feeds y Flour Mill Ltd, Ewu, Estado de Edo, Nigeria y se les proporcionó agua libremente. Las ratas fueron sacrificadas en el día cuarto, octavo y décimo quinto de la prueba. El cuerpo geniculado medio fue cuidadosamente disecado y rápidamente fijado en el 10% de solución de formol salina para el estudio histológico.
Los hallazgos histológicos observados en secciones del cuerpo geniculado medio, teñidas con H&E indicaron que el tratamiento disminuye la población celular, con cambios degenerativos, hipertrofia celular, y aparecen en el estroma algunas vacuolizaciones.
Variando la dosis y tiempo de administración de artesunate, pueden verse algunos efectos perjudiciales sobre las neuronas del cuerpo geniculado medio y pueden probablemente tener algunos efectos negativos sobre la sensibilidad auditiva por sus efectos nocivos sobre las células del cuerpo geniculado medio de las ratas wistar adultas. Por consiguiente, se recomienda realiar nuevos estudios para corroborar estas observaciones.
Palabras Clave: Artesunate, cuerpo geniculado medio, disminución de poblacion celular, vacuolazion.
INTRODUCTION
Malaria remains one of the world's most significant health problems despite increasing research and control efforts1. The occurrence of malaria during pregnancy exposes the mother and infants to serious risks. It is therefore imperative that pregnant women be protected against malaria; and that pregnant women with malaria receive treatment as soon as possible2.
Artesunate is one of the numerous drugs for malaria intervention in Nigeria. It is a semi synthetic derivative of artemisinin, the active compound of the Chinese herb Artemisia annua which consist of the sodium succinyl salt of dehyroartemisinin3. Artemisinin-type compounds reduce malaria parasitemia more rapidly than any other known antimalarial drugs and are effective against multi drug resistant malaria parasites4-5. Artesunate is highly effective against multi-drug resistant strains of plasmodium falciparum hence its increasingly wide usage for the treatment and management of malaria6. Artesunate is well tolerated at therapeutic doses; therefore a lot of people, pregnant women inclusive take the drug.
Several studies have shown that high doses of artesunate can produce neurotoxicity such as selective damage to brainstem centres in mice and rats7-9. Artesunate have been reported to cause gait disturbances, loss of spinal cord and pain response mechanisms in animals10-11.
The Inferior colliculus and Medial geniculate body constitute the intracranial auditory relay centres. The medial geniculate body is the target of ascending projections from the inferior colliculus and descending input from the auditory cortex; this is the obligatory synaptic target in the thalamus for hearing. It contains interleaved and overlapping tonotopic and aural bands12. The cerebral cortex strongly affects the medial geniculate body through descending projections which are thought to consist primarily of small areas with slow conduction velocities13.
Cerebral nuclei such as the medial and lateral geniculate bodies, inferior and superior colliculi have higher glucose utilization than other structures. There is also a correlation between functional activity and metabolic rate such as in the visual and auditory system14.
The effects of artesunate on the intracranial auditory relay centre may not have been documented, but there have been reports that it may be implicated in varied symptoms of dizziness, itching, vomiting, abdominal pain, headaches, diarrhea, tinnitus, increase hearing loss, macular rash, neutropenia and convulsion. It is probable that the adverse effects of artesunate on hearing such as tinnitus may be due to direct effect of artesunate on this auditory relay centre. This present study was to elucidate the histological effects of artesunate on the medial geniculate body of adult wistar rats.
MATERIALS AND METHODS
ANIMALS:
Twenty-four (24) adult wistar rats of both sexes with average weight of 210g were randomly assigned into four groups A, B, C and D of (n=6) in each group. Groups A, B, and C of (n=18) serves as treatments groups while group D (n=6) is the control. The rats were obtained and maintained in the Animal holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Nigeria. They were fed with grower's marsh obtained from Edo feed and flour mill limited, Ewu, Edo state) and were given water liberally. The rats were allowed to gain maximum acclimatization before the actual commencement of the experiment. The Artesunate tablets were obtained from the University of Benin Teaching Hospital Pharmacy, Benin City, Edo state, Nigeria.
ARTESUNATE ADMINISTRATION:
The rats in the treatment groups (A, B, & C) received 4mg/kg body weight of Artesunate base dissolved in distilled water for the first day. Animals in the treatment group 'A' continued with this dosage for the next two days, while animals in groups B & C received 2mg/kg once daily for six and thirteen days respectively. The control group D received equal volume of distilled water using orogastric tube. The treated rats in groups A, B, and C were sacrificed by cervical dislocation on the 4th, 8th and 15th day of the experiment respectively, while that of the control group D was sacrificed at the end of the experiment. The skulls were opened using bone forceps to expose the brain of the rat, and the medial geniculate body was quickly dissected out and fixed in10% formal saline for routine histological techniques.
HISTOLOGICAL STUDY:
The tissue were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7 microns thick were obtained using a rotatory microtome. Some of the deparaffinised sections were stained routinely with haematoxyline and eosin (H&E) method15. The digital photomicrographs of the desired sections were made in the Department of Anatomy research laboratory, University of Benin, Nigeria for further observations.
RESULTS
The sections of the medial geniculate body (MSG) from the control group showed normal histological features with the neurons appearing distinct and the glial cells normal without vacuolation in the stroma (Figure 1).
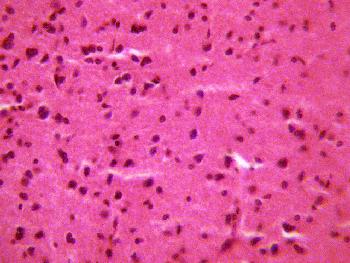
Figure 1 (GROUP D): Photomicrograph representing the control section
of the medial geniculate body (Original magnificacion x 400).
The sections of the medial geniculate body from the treatment (A, B, & C)
groups showed some decrease in cellular population, degenerative changes,
cellular hypertrophy and vacuolations appearing in the stroma (Figure 2, 3 &4).
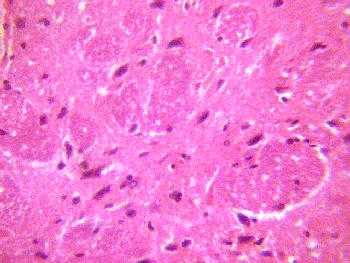
Figure 2 (GROUP A): Photomicrograph representing the treatment section of the medial geniculate body (group A),
that received 4mg/kg of artesunate for 3 days. (Original magnificacion x 400).
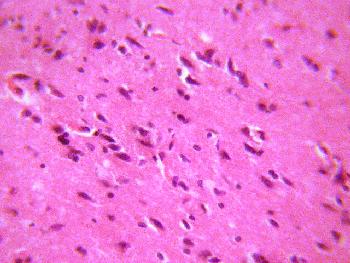
Figure 3 (GROUP B): Photomicrograph representing the treatment section of the medial geniculate body (group B)
that received 4mg/kg 1st day and thereafter 2mg/kg for 6 days of artesunate. (Original magnificacion x 400).
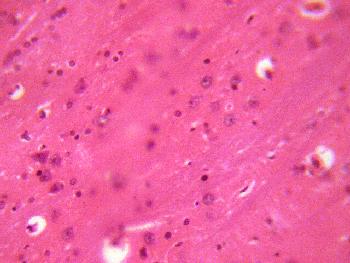
Figure 4 (GROUP C): Photomicrograph representing the treatment section of the medial geniculate body (group C),
that received 4mg/kg 1st day and thereafter 2mg/kg for 13 days of artesunate. (Original magnificacion x 400).
All the rats in each group were affected equally, with the rats in group C more
severe than groups A and B. Affectation of the rats in group A was mild and
Group B appears to be moderate.
DISCUSSION
The results (H & E) revealed that administration of artesunate showed some decreased cellular population, degenerative changes, cellular hypertrophy and vacuolations which appeared in the stroma of the treatment groups compared with the control section of the medial geniculate body. Neuronal degeneration has been reported to result in cell death, which is of two types, namely apoptotic and necrotic cell death. These two types differ morphologically and biochemically16. Pathological or accidental cell death is regarded as necrotic and could result from extrinsic insults to the cell such as osmotic, thermal, toxic and traumatic effects17. It was reported that cell death in response to neurotoxins might trigger an apoptotic death pathway within brain cells18. Cell death in response to neurotoxins occurs as a controlled event involving a genetic programme in which caspase enzymes are activated18.
The process of cellular necrosis involves disruption of the membranes structural and functional integrity. Cellular necrosis is not induced by stimuli intrinsic to the cells as in programmed cell death (PCD), but by an abrupt environmental perturbation and departure from the normal physiological conditions19. There is the need to further investigate the actual mechanism by which artesunate induced neuronal degeneration in the medial geniculate body of adult wistar rat in this study.
Extensive cell death in the central nervous system is present in all neurodegenerative diseases18. The type of nerve cell loss and the particular part of the brain affected dictate the symptoms associated with an individual disease18. In this study artesunate may have acted as toxin to the cells of the medial geniculate body, affecting their cellular integrity and causing defect in membrane permeability and cell volume homeostasis.
In cellular necrosis, the rate of progression depends on the severity of the environmental insults. The greater the severity of the insults the more rapid the progression of neuronal injury19. The principle holds true for toxicological insult to the brain and other organs20. The prime candidates for inducing the massive cell destruction observed in neurodegeneration are neurotoxins18. These may be substances present in small amounts in the environment, or even naturally occurring chemicals such as glutamate used by the brain as transmitter's substances18. The latter when present at a critical level can be toxic to the brain cells they normally excite18. It is inferred from this results that prolonged and high dose of artesunate resulted in increased toxic effects on the MGB. The decrease in cellular population observed in this study may have been as a result of cell death caused by the toxic effect of artesunate. In the same way, it has been reported that chronic administration of chloroquine resulted in the cellular degenerative changes, sparse cellular population and vacuolation appearing in the stroma with some autophagic vacuoles in the medial geniculate body and inferior colliculus of adult wistar rats21-22. Chloroquine intoxication has been reported to result in the accumulation of lysosomal membranes rich in phospholipids and gangliosides due to the inhibition of lysosomal enzymes23.
The vacuolation observed in the stroma of the medial geniculate body in this experiment may be due to artesunate interference, since it has been reported that artesunate may be neurotoxic to the developing nervous system of wistar rats24. The cellular hypertrophy observed in this experiment may be due to the adverse effects of artesunate on the medial geniculate body. This study may underlie the possible neurogenic symptoms such as dizziness and tinnitus for high doses of artesunate has been reported to produce neurotoxicity such as selective damage to brainstem centres in mice and rats7-9.
CONCLUSION
Our study revealed that high doses and long term administration of artesunate causes spares cellular population, cellular degenerative changes, cellular hypertrophy and vacuolation in the medial geniculate body of adult wistar rats. These results may indicate that the functions of the medial geniculate body in auditory sensibility may be adversely affected.
REFERENCES
1. Curtis CF. Workshop on bed net at the international congress of Tropical Medicine; JPM Saint Zool. 1993; 2:63-68.
2. WHO. Reproductive Risk Assessment of Antimalaria therapy with Artemisinin compounds- report of an informal consultation convened by WHO Geneva. 2002; May 29-30.
3. Ittarat WR, Udomsangpeth KT, Chotivanich, Looareesuwan S. The effects Of quinine and Artesunate treatment on plasma tumor necrosis factor levels in malaria infected patients. SEAMEO TROPMED. 1999; 30:7-10.
4. Meshnick SR, Taylor TE, Kanchonwongpaisan P. Artemicinin and the Antemalaria endoperoxidase: From herbal remedy to targeted chemotherapy; Microbiol Res. 1996; 60: 301-315
5. OIliaro PL, Haynes RK, Meunier B, Yuthavong Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001; 17: 122-126.
6. Van Agtmed MA, Eggette TA, Van Boxtel CJ. Artemisinin drugs in the treatment of malaria: From medicinal herb to registered medication. Trends Pharmacol Sci. 1999; 20: 199-205
7 Nontprasert A, Pukrittayakamee S, Nosten-Bertrand M, Vanijanonta S. Assessment of neurotoxicity of parenteral artemisinin derivatives in mice. Am J Trop Med Hyg. 1998; 59: 519-522
8 Genovese RF, Newman DB, Brewer TG. Behavioral and neural toxicity of the artemisinin antimalaria arteether, but not artesunate and artelinate in rats. Pharmacol Biochem Behav. 2000; 67: 37-44
9 Nontprasert A, Pukrittayakamee S, Dondorp AM, Clemens R, Looareesuwan S, White NJ. Neuropathologic toxicity of artemisinin derivatives in a mouse model. Am J Trop Med Hyg. 2002; 67: 423-429.
10 Genovese RF, Petras JM, Brewer TG.Arteether neurotoxicity in the absence of deficits in behavioral performance in rats. Ann. Trop. Med. Parasitol 1995; 89: 447-449
11. Dayan AD. Neurotoxicity and Artemisinin compounds: Do the observations in animals justify limitations in clinical use? Paper presented at a conference convened by the international Larveran Association. Annecy, France April 1998; 19-22
12. Fall. Mammalian Neuroanatomy MCB 163: 1999
13. Winner JA, Saint Marie RL, Larue DT, Oliver DL. The cerebral cortex strongly affects the medial geniculate body through descending projections. Proc. Nat Acad. Sci, USA 1996; 93: 8005-8010
14. Siesjo BK. Utilization of substrates by brain tissues. Brain energy metabolism. John Wiley and Sons, USA. 1978; 101-130.
15 Drury RAB, Wallington EA, Cameron R. Carleton's Histological Techniques: 4th ed., Oxford University Press NY. USA. 1967; 279-280.
16 Wyllie AH. Glucocorticoid-induced thymocyte apoptosis in associated and endogenous endonuclease activation. Nature. 1980; 284: 555-556.
17 Farber JL Chein K R, Mittnacht S. The pathogenesis of Irreversible cell injury in ischemia; Am J Pathol 1981; 102: 271-281
18 Waters CM. Glutamate induced apoptosis of striatal cells in rodent model for Parkinsonism. Neuroscience 1994; 63: 1-5
19. Ito U, Sparts M, Walker JR, Warzo I. Experimental Cerebral Ischemia in Magolian Gerbils(1). Light microscope observations. Acta Neuropathol. USA 1975; 32: 209-223.
20. Martins LJ, Al-Abdulla NA, Kirsh JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischaemia and target Deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 1978; 46: 281-309.
21. Adjene JO, Caxton-Martins A. Some histological effect of chronic administration of Chloroquine on the medial geniculate body of adult wistar rat Afri J Med Sci 2006; 35: 131-135.
22 Adjene JO, Adenowo TK. Histological studies of the effect of chronic administration of Chloroquine on the inferior colliculus of adult wistar rat. JMBR 2005; 4: 83-87
23 DeGroot PG, Elferenk RO, Mariastruland A, Westeveld A, Khan PM, Tager JM. Inactivation by chloroquine of a-galactosidase in cultured humanskin fibroblast. Expr Cell Res. 1981; 36: 327-333
24 Mesembe OE, Ivang AE, Udo-Attah G, Igiri AO, Fischer VA, Akpaso M, Eluwa MA, Akpa OA. A morphometric study of the teratogenic effect of Artesunate on the central nervous system of the Wistar rats foetus. Nig J Physiol Sci. 2004; 19: 92-97
Corresponding author
Dr. A. O. Eweka
Department of Anatomy. School of Basic Medical Sciences. College of Medical Sciences, University of Benin.
Benin City. Edo State. Nigeria.
andreweweka @ yahoo.com
Received June 28, 2007. Received reviewed November 19, 2007
Published February 20, 2008
