Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
SIMPLE AND SENSITIVE METHOD FOR DETERMINATION OF POLYHEXANIDE IN MULTIPURPOSE SOLUTION
A. Arora1, A. Ali2, M. T. Zzaman1,
1 KIET School of Pharmacy, Ghaziabad.
2 Jamia Hamdard, Faculty of Pharmacy, Dept of Pharmaceutics. New Delhi.
India.
alkamailin @ yahoo.co.in
Rev Electron Biomed / Electron J Biomed 2009;2:30-37
Comment of the reviewer Silvia Albillos García PhD. Inbiotec (Instituto de Biotecnología). León, España
Comment of the reviewer Mónica Cavia Sáiz CD. Research Unint. Complejo Asistencial de Burgos. Burgos. España
RESUMEN: MÉTODO SENCILLO Y SENSIBLE PARA LA DETERMINACIÓN DE POLIHEXANIDE EN SOLUCIÓN MULTIUSO.
Un método invertido sencillo de fase HPLC ha sido desarrollado para la determinación de clorhidrato biguanudo de poli-hexametileno en la solución multiuso para las lentillas hidrófilas. La fase móvil fue acetonitrile 1%(v/v) acetato amónico de 20 mmol/L en el agua como 16: 84 (V/V) en una tasa de flujo de 1 ml/min. El clorhidrato biguanudo de poli-hexametileno fue detectado por la absorción de UV a 235 nm. El pH fue mantenido a 4,0 utilizando el ácido acético glacial. El método de Yiping et al ha sido modificado ligeramente según la necesidad. La cantidad detectada es 2 µG/ml y por eso fue llevado a cabo utilizando el método de pre concentración bajo vacio. Desde el cromatograma, fue observado que un pico claro apareció al tiempo de retención 5,883 min para el clorhidrato biguanudo de poli-hexametileno. La recuperación de droga fue encontrada a 99,38% y el método fue sencillo, rápido y propio para la concentración de la droga en la solución multiuso para las lentillas hidrófilas y para el llevar a cabo la estabilidad según la directriz de ICH para valorar la estabilidad de clorhidrato biguanudo de poli-hexametileno en la solución multiuso.
PALABRAS CLAVE: Líquido estándar de lágrima (LEL). Clorhidrato de Polihexanide (CHPH). Área bajo curva (ABC)
SUMMARY:
A simple reversed phase HPLC method has been developed for the determination of polyhexamethylene biguanide hydrochloride in multipurpose solution for hydrophilic contact lenses. The mobile phase was acetonitrile 1% (v/v) ammonium acetate 20 mM in water as 16: 84 (v/v) at a flow rate of 1 ml / min. Polyhexamethylene biguanide hydrochloride was detected by UV absorption at 235 nm. The pH was kept at 4.0 using glacial acetic acid. The method of Yiping et al has been slightly modified as needed. The quantity detected was 2 µg/ml so it was carried out using a preconcentration method under vaccum. From the chromatogram, it was observed that a distinct peak appeared at retention time 5.883 min for polyhexamethylene biguanide hydrochloride. The recovery of drug was found to be 99.38% and the method was simple, rapid and suitable for the assay of drug in multipurpose solution for hydrophilic contact lenses and for carrying out stability as per ICH guidelines to assess the stability of polyhexamethylene biguanide hydrochloride in multipurpose solution.
KEY WORDS: Standard tear fluid (STF). Polyhexanide hydrochloride (PHNB).
Area under curve (AUC)
INTRODUCTION
Polyhexamethylene biguanide hydrochloride (PHMB.HCL) has been utilized as a new antimicrobial providing reliable preservation in multipurpose solution (MPS) for hydrophilic contact lenses1,2. It is a polymeric compound with low skin irritancy, low eye toxicity, fast speed of kill, stable and effective over a wide pH range. It is available as 20% aqueous solution (w/v) with a molecular structure as shown in figure 1.
The composition of MPS is given in table 1. The MPS is found to be effective against bacteria, fungi, protozoa and viruses even at very low concentration3,4.

In order to supervise the quality of MPS containing PHMB.HCl, it is necessary to develop the method to assay the sample of drug accurately. Reversed phase high performance liquid chromatography (RP-HPLC) with photodiode array detector (PDA) is the most frequently used method for routine determination. However, to the best of our knowledge, no RP-HPLC method for the analysis of PHMB.HCl in MPS for hydrophilic contact lenses has been reported except that of Yiping et al.5 who has tried to establish a simple and rapid HPLC method for the routine analysis of PHMB in compound chemical disinfection.
MATERIALS AND METHODS
Materials
PHMB. HCl was obtained from Avecia Biocides, Manchester, U.K. Acetonitrile and water HPLC grade were from S.D. chemicals, Mumbai, India. Ammonium acetate, glacial acetic acid were of analytical grade and were used as received.
HPLC instrumentation and chromatographic conditions
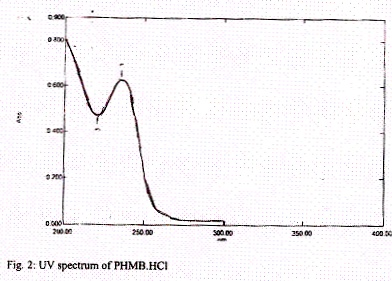
HPLC was performed with a Shimadzu class VP series manual injector fitted with a 20 µl loop, a model SPD- 10 UV detector, a model LC- ATVP pump operated in an isocratic mode and a 4.6 mm i.d sign 50 mm Shim- pack CLC- ODS C18 column, particle size 5 µm. The mobile phase was 16:84 (v/v) of acetonitrile -1% (v/v) and ammonium acetate 20 mM in water and it was pumped out at 1 ml / min. The mobile phase was filtered through a 0.45 µm membrane filter and ultrasonified before use. The UV absorption spectrum of PHMB.HCl indicated the presence of an analytically useful absorption band with a maximum at 235 nm as shown in figure 2.
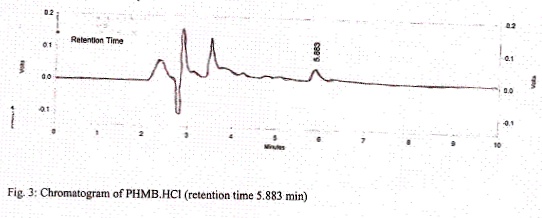
The 20 µl samples of MPS which was pre-concentrated under vacuum was injected and chromatogram was obtained. The temperature was maintained at 30 °C. Under this condition, PHMB.HCl was eluted at 5.883 min as shown in figure 3. Interferences from other ingredients of MPS were not observed.
Preparation of Standard Curve
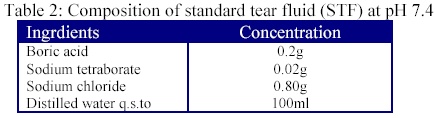
From the drug sample (20% w/v), 1.0 ml was pipetted and transformed into 100 ml capacity volumetric flask and volume was made up to 100 ml with filtered standard tear fluid (STF) of pH 7.4. The composition of STF was as mentioned in table 2.
From this solution 1.0 ml was further diluted up to 100 ml with the STF of pH 7.4 in a 100 ml volumetric flask in order to obtain a stock solution of 20 µg /ml. From this stock solution different volumes were withdrawn and diluted with STF of 7.4 for obtaining different concentration from 0.25 µg /ml to 7.5 µg /ml. The volume of each standard solution was 10 ml and all these were filtered through a membrane filter (0.45 µm). The 20 µl of each solution was injected into the HPLC column in order to obtain the chromatogram and AUC (area under the curve) was calculated. The observed values are given in table 3.
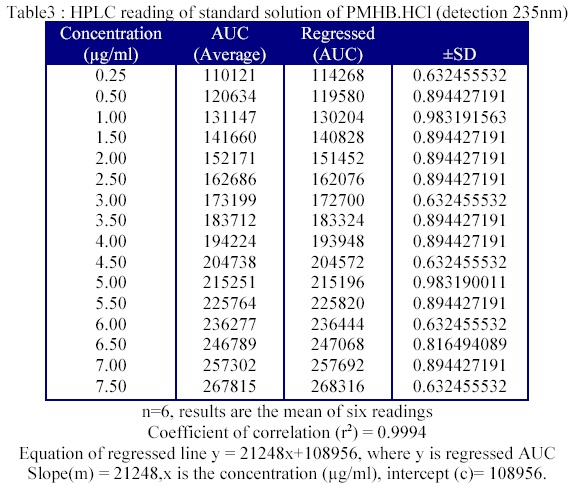
The method was repeated for six times i.e. n=6. A standard plot PHMB. HCL was drawn by plotting concentration on X- axis and AUC on Y- axis and is shown in figure 4.
From the point observed the regressed line was drawn and the equation of this regressed line was calculated. The coefficient of correlation (r2) was also determined. The coefficient of correlation (r2) = 0.9994 and equation of regressed line y= 21248x+ 108956, where y is regressed AUC and slope (m) is 21248, x is the concentration (µg / ml) and intercept (c) is 108956.
Determination of PHMB.HCl in MPS under Stability Studies as per ICH Guidelines
Under the stability studies of the formulation, 20 µl of the sample undiluted were injected into an HPLC column, using similar conditions as in the case of the standard curve and AUC was obtained. The drug concentration obtained by extrapolation of the AUC of the sample under the standard curve and the potency of the drug in the sample was calculated.
Sample Stability
The Stability studies were carried out according to the ICH guidelines and for this purpose MPS which were developed on pilot scale were kept at 40°C and 75% RH in humidity chamber for 6 months. The samples were withdrawn at intervals of 0, 1, 2, 4 and 6 months and analyzed for drug content by the HPLC method of analysis as mentioned before. The results are given in table 4.
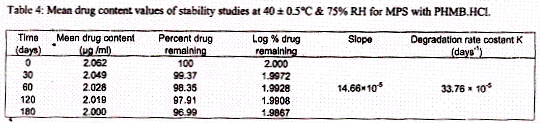
The results of the stability studies carried out as per ICH guidelines were compared with those of the sample at 0 day. The data converted into log percent drug remaining and it was plotted against time in days as shown in figure 5.
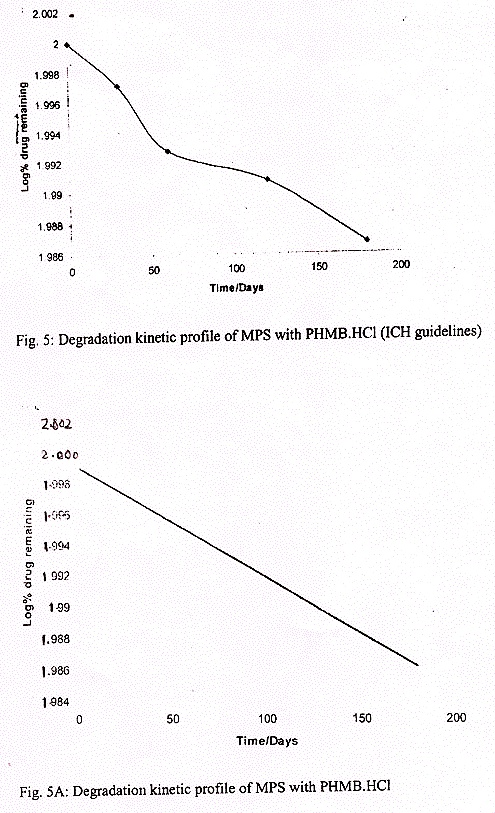
The slope of the line was determined, and from this, the degradation rate constant 'K' in days was calculated.
RESULTS AND DISCUSSION.
An HPLC method of analysis of PHMB. HCl was carried using the method of Yiping et al after slight modification. Under the method, UV detection at 235 nm was performed. The UV spectrophotometric determination was carried out in isotonic STF of pH 7.4. For this purpose, the drug solution was scanned in UV range and PHMB.HCl exhibited 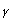 max at 235 nm. The drug was detected accurately with recovery of 99.38%. The standard curve under HPLC analysis between AUC and concentration obeyed Lambert's Beer law between the concentration of 7.5 µg/ml and a high value for the coefficient of correlation was observed. This method of analysis was found to be simple, rapid and accurate for the determination of PHMB.HCl. The sample was preconcentrated by vaccum before subjecting it to HPLC studies.
max at 235 nm. The drug was detected accurately with recovery of 99.38%. The standard curve under HPLC analysis between AUC and concentration obeyed Lambert's Beer law between the concentration of 7.5 µg/ml and a high value for the coefficient of correlation was observed. This method of analysis was found to be simple, rapid and accurate for the determination of PHMB.HCl. The sample was preconcentrated by vaccum before subjecting it to HPLC studies.
Stability studies were carried out as per ICH guidelines in order to see the stability of drug in the MPS. Under the ICH methodology, sufficient number of containers of the product were kept at 40 ± 0.5 °C and RH 75% in a humidity chamber for six months. The samples were withdrawn at different time intervals and analyzed for the drug (PHMB.HCl) by the HPLC method of analysis. The log percent of drug remaining was plotted against time in days and from the curve, the slope was determined. By using the slope, the degradation rate constant was determined and it was found to be 33.76×10?5 days?1. The amount of drug degraded was less than 5 % of the total. Hence an arbitrary shelf life of two years can be assigned to the product as mentioned by ICH guidelines.
CONCLUSIONS
The HPLC method with slight modification of Yiping et al was found to be precise, accurate and suitable. The method was found to be suitable to fix an adequate shelf life for the product.
REFERENCES
- 1. John T, Desai D, Sahm D. Adherence of Acanthamoeba castellanii cysts and trophozoites to unworn soft contact lenses. Am J Ophthalmol. 1989;108:658-664.
2. Silvany RE, Dougherty JM, McCulley JP, Wood TS, Bowman RW, Moore MB. The effect of currently available contact lens disinfection systems on Acanthamoeba castellanii and Acanthamoeba polyphaga. Ophthalmology. 1990;97:286-290.
3. Levey SB, Cohen EJ. Methods of disinfecting contact lenses to avoid corneal disorders. Surv Ophthalmol. 1996;41:245-251
4. Lever AM, Miller MJ. Comparative antimicrobial efficacy of multi-purpose lens care solutions using the FDA's revised guidance document for industry: stand-alone primary criteria. CLAO J. 1999;25:52-56.
5. Yiping C, Xiaojing D, Pengyani. Rapid determination of Polyhexanide in compound chemical disinfectants by reversed phase HPLC. 2005;8(7):1-5
