Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
Letters to the Editor / Cartas al Editor
THE CHICK CHORIOALLANTOIC MEMBRANE
AS A MODEL TISSUE FOR
IRRITATION STUDIES
ON MULTIPURPOSE SOLUTION
A. Arora1, A. Ali2, M. T. Zzaman1, S.Chauhan1
1 KIET School of Pharmacy, Ghaziabad.
2 Jamia Hamdard, Faculty of Pharmacy, Dept of Pharmaceutics. New Delhi.
India.
alkamailin @ yahoo.co.in
Rev Electron Biomed / Electron J Biomed 2009;2:79-84
To the Editor:
The chick chorioallantic membrane (CAM), a part of the extraembroynic tissue begins to develop 7 days after initial incubation from the fusion of the chorion and the allantois. Structurally, the outer epithlial layer of the chorion is derived from the trophoblast, which opposes the allantois. This structure forms a supportive matrix for the extensive vascular network that courses through the CAM, analogus to the retina and its vasculture. Overall mature chick CAM (20-100 µm) and human retina (approx 100-300 µm) are of roughly comparable thickness. Mature chick CAM (incubation day 12 and on) can be divided into three anatomically distinct layers. 1.- Primary stratum, 2.- Capillary plexus or blood sinus, 3.- A thin stratum.
The specialized chorionic epithelial cells that have presumably migrated above the capillary plexus are involved in gas exchange and calcium absorption. Immediately above and attached to CAM thin layer is the inner shell membrane1-3.
The irritation study was performed in order to ascertain the irritating effect if any of multipurpose solution (MPS) used for rinsing and soaking of hydrophilic contact lenses (CL's) before being placed into the eyes. In the present study CAM test was used for performing irritation studies on MPS used for hydrophilic CL's. The CL's are to be rinsed and soaked in multipurpose solution (MPS) before being worn. The composition of MPS coded as MPS-2 is given in table 1. In a fertile egg, the CAM test has been used extensively in the past for ocular angiogenesis studies. The tissue is a useful tool for ocular irritation studies including hyperaemia, hemorrhage and coagulation.
The CAM test is a convenient model as it involves low cost and hence considered less expensive than the living animal. The eggs are maintained at adequate humidity at 37 + 0.5oC temperature condition and rotated after every few hours. These conditions can be maintained in an incubator and the eggs of 14 days old are fit for experimental work. The CAM experiment can be performed between 12-17 days of incubation. Before 12 days, the CAM vasculture is not adequately matured and after 17 days the embryo is large enough and hence its size and movement underneath the CAM could disturb the experimental maneuvers on the CAM hence 12-14 days of incubation is the ideal time for experimental work4-8.
The test is based on the scoring scheme for hyperaemia, hemorrhage and coagulation parameters. Any increase in red color of CAM (hyperaemia) can be given a score number from 0 to 5. Similarly the haemorrhage (leaking of blood) can be given a score from 0 to 7 i.e. no haemorrhage at 0 and strong haemorrhage at 7. Scores from 0 to 9 is used for coagulation parameter. A detailed account is given in table 2.
The cumulative scores were used for assessment of extent of irritation and taking hyperaemia, haemorrhage and coagulation into consideration as given below9-13. Cumulative scores and irritation assessment: 0 - 0.9 practically none; 1 - 4.9 slight; 5 - 8.9 moderate; 9 - 21.0 strong.
Multipurpose solution (MPS-2) prepared on pilot scale at Gaymed labs Delhi, India. Propylene glycol and Sodium lauryl sulphate from E Merck, Mumbai, India. Baby shampoo from Johnson & Johnson Mumbai. All other materials were used as received.
Fertilled chick eggs are easily ordered and procured from poultry farm and do not require an extensive animal protocol (as long as they are used and disposed before 19 days). These are considerably less expensive than the living animals currently used in ophthalmic and contact lens experimentation (e.g. rabbits, rats, cats, minipigs, dogs). Maintenance of eggs with 75% of relative humidity, a 37°C environment and rotation after 2 hours, can be performed in an inexpensive incubator.
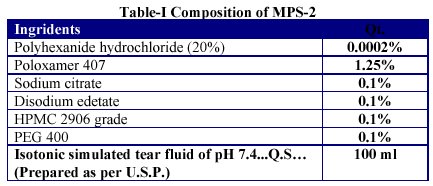
Immediately after procurement the fertilized chick eggs (12 days old) were washed with water and kept on cotton surface already placed in a tray. The tray was kept in an incubator for 2 days at 37 ± 0.5 °C. The eggs were rotated after every 2 hours (figure 1).
Figure 1.- One of the egg in the tray
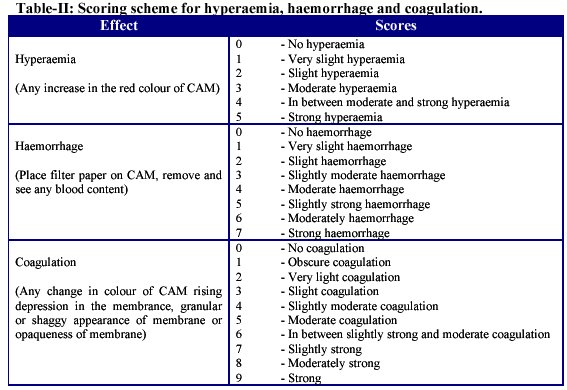
To prepare CAM for typical experiment, the eggs were examined under candle light and only healthy and fertile eggs were selected. The egg shell is cracked and peeled away from the region over the air space that exists between the shell and the inner shell membrane (ISM) at one pole of the egg (figure 2).
This air space can be visualized before the egg is cracked by holding the egg under intense light. The eggs were cracked. The white part of the egg was removed carefully with the help of a pointed needle and a sharp blade in order to expose, once the opaque CAM-ISM dual layer is exposed, the irrigation with saline 0.9% will cause the dual layer to become transluscent, allowing for visualization of the CAM vasculture as shown in (figure 3).
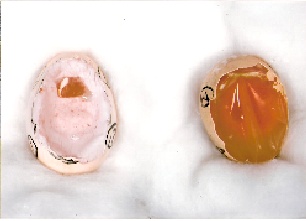 Figure 2.- The cracked egg shell.
|
 Figure 3.- The CAM of Hen’s fertile egg |
The exposed CAM was divided into four parts 1, 2, 3 and 4. These were written on the four sides.The preparations were applied on these spots as a drop in the following manner: Area 1: Multipurpose solution coded as MPS-2. Area 2: Sodium lauryl sulphate (SLS). Area 3: Baby shampoo (Johnson). Area 4: Propylene glycol (PG)
Immediately, these were kept in the incubator and after 0.5, 2 and 5 minutes, the area were examined for hyperaemia, haemorrhage and coagulation. The resultant scores obtained were shown in table 3
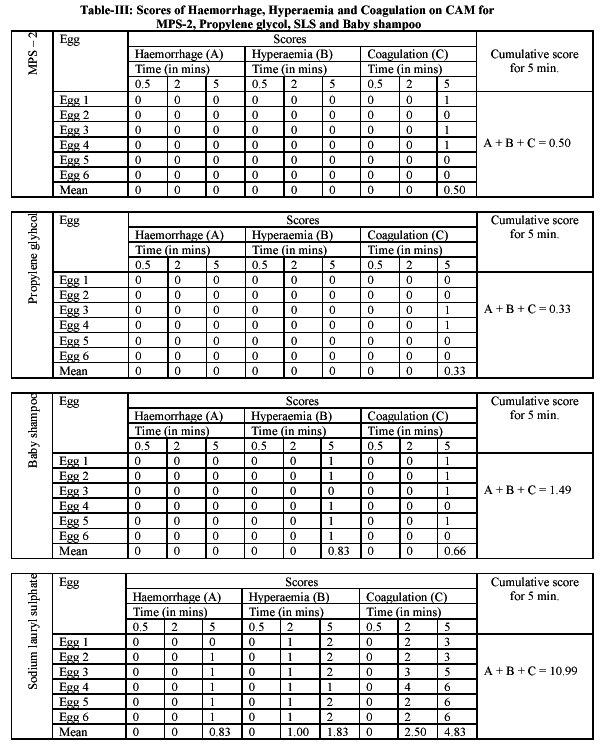
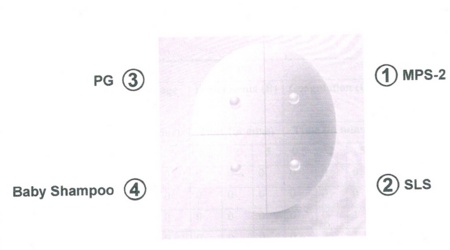
Figure 4.- Diagram Showing the Scheme of Application on CAM Sample.
The multipurpose solution coded as MPS-2 was formulated on pilot scale. The irritation studies on the preparation MPS-2 used as rinsing and soaking solution for hydrophilic contact lenses were performed using chorio allantoic membrane (CAM) test of fertile hen's egg. The preparation was applied as a drop on the CAM and scores for haemorrhage, hyperaemia and coagulation were observed and were recorded. The mean score was calculated and cumulative score was obtained. This was obtained as 0.50.As per the interpretation of standard parameter a cumulative score in between 0-0.9 is practically non-irritant. Therefore multipurpose solution MPS-2 was found to be practically non-irritant. For comparative purpose the test was also performed for propylene glycol, baby shampoo and sodium lauryl sulphate. For propylene glycol the cumulative score was 0.33 hence it is also nonirritant. This was practically observ.ed on CAM. A substance is slightly irritant if the mean score is in the range of 1-4.9. For sodium lauryl sulphate, the cumulative score was 10.99 hence it indicated a strong irritation effect as it was in the cumulative score range of 9-21. The three materials that were used. Propylene glycol, baby shampoo and SLS as controls in the study.
Irritation studies should be carried out on preparation like multipurpose solution because it has to be used for rinsing and soaking of CL's (hydrophilic contact lenses). The CAM is very much similar to retina of the eye hence can be used for irritation studies on eye preparations. Such experiments are less expensive and do not require extensive animal protocol. Maintenance of eggs requires adequate humidity and a 37°C environment and rotation after every few hours, which can be performed in an inexpensive incubator.
The type of CAM experiments described herein can optimally be performed between incubation days 12 and 18. Before day 12, the CAM vasculture has not adequately matured. After day 18, the embryo is large enough that its size and movements underneath the CAM can disrupt experimental maneuvers on the CAM, Additionally, standard animal protocols for chick embryos past 18 require more complicated euthanasia techniques.
Conclusion: The optimized product MPS-2 and marketed by the name of Multisol is practically non irritant and can be used for rinsing, soaking, disinfecting, lubricating and deproteinising hydrophilic contact lenses.
REFERENCES
-
1.- Ribatti D, Nico B, Vacca A, Roncali L, Burri PH, Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo.
Anat Rec. 2001;264:317-324.
2.- Oncel M, Peyman GA, Khoobehi B. Tissue plasminogen activator in the treatment of experimental retinal vein occlusion. Retina. 1989;9:1-7.
3.- Leng T, Miller JM, Bilbao KV, Palanker DV, Huie P, Blumenkranz MS. The chick chorioallantoic membrane as a model tissue for surgical retinal research and simulation. Retina. 2004;24:427-434.
4.- Thompson WD, Reid A. Quantitative assay for the chick chorioallantoic membrane. Adv. Exp Med Biol 2000; 476:225-236
5.- Ponce ML, Kleinmann HK. The Chick chorioallantoic membrane as an in vivo angiogenesis model. Curr Protoc Cell Biol 2003;Chapter 19:Unit 19.5
6.- Liao Y, Wang X, Zhang L, Li G. Study on using the hen's egg test-chorioallantoic membrane as an alternative method of draize eye irritation test. Wei Sheng Yan Jiu. 2004;33:279-283.
7.- Debbasch C, Ebenhahn C, Dami N, Pericoi M, Van den Berghe C, Cottin M, Nohynek GJ. Eye irritation of low-irritant cosmetic formulations: correlation of in vitro results with clinical data and product composition. Food Chem Toxicol. 2005;43:155-165.
8.- Tufan AC, Satiroglu-Tufan NL. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr Cancer Drug Targets. 2005;5:249-266
9.- Tavaszi J, Budai P. Toxicity study of agrochemicals on chorioallantoic membrane of the egg. Commun Agric Appl Biol Sci. 2006;71(2 Pt A):101-5.
10.- Tavaszi J, Budai P. The use of HET-CAM test in detecting the ocular irritation. Commun Agric Appl Biol Sci. 2007;72:137-141.
11.- Deryugina EI, Quigley JP. Chapter 2. Chick embryo chorioallantoic membrane models to quantify angiogenesis induced by inflammatory and tumor cells or purified effector molecules. Methods Enzymol. 2008;444:21-41.
12.- Slodownik D, Grinberg I, Spira RM, Skornik Y, Goldstein RS. The human skin/chick chorioallantoic membrane model accurately predicts the potency of cosmetic allergens. Exp Dermatol. 2009;18:409-413.
13.- Deryugina EI, Quigley JP. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol. 2008;130:1119-1130
Prof. Alka Arora PhD
3- B-264 Derawal Nagar, opp. Model Town-2 Delhi-110009, India.
mail alkamailin @ yahoo.co.in
Received reviewed, July 1, 2009.
Published, July 19, 2009
