Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HISTOLOGICAL EFFECTS OF CHRONIC CONSUMPTION OF NUTMEG ON THE LATERAL GENICULATE BODY OF ADULT WISTAR RATS.
J.O. Adjene* and P.S. Igbigbi**
*Department of Anatomy, School of Basic Medical Sciences.
University of Benin, Edo State.
**Department of Anatomy, Faculty of Basic Medical Sciences.
Delta State University, Abraka.
Nigeria
joadjene @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2010;1:33-38
Comment of the reviewer José María Trejo Gabriel y Galán PhD. Head of Dept. of Neurology. Complejo Asistencial Universitario de Burgos. España.
Comment of the reviewer Prof. Pilar Muñiz Rodríguez PhD. Biochemistry and Molecular Biology. Sciences Faculty. Universidad de Burgos. España
ABSTRACT
The effects of chronic consumption of nutmeg commonly used as a spice in various dishes, as components of teas and soft drinks or mixed in milk and alcohol on the lateral geniculate body of adult wistar rats was studied.
The rats of both sexes, with average weight of 200g were randomly assigned into treatment and control groups. The rats in the treatment group (n=8) received 2g of nutmeg thoroughly mixed with the feeds on a daily basis for thirty-two days. The control group (n=8) received equal amount of feeds daily without nutmeg added for thirty-two days. The growers mash feeds was obtained from Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria and the rats were given water liberally. The rats were sacrificed on the thirty-three day of the experiment. The lateral geniculate body was carefully dissected out and quickly fixed in 10% formal saline for histological study.
The findings indicate that rats in the treated group showed some cellular degenerative changes like sparse cellular population, pyknotic nuclei with some microcystic changes, edema and vacuolations in the stroma of the treated lateral geniculate body as compared to that of the control group.
Chronic consumption of nutmeg may therefore have an adverse effect on the visual sensibilities by affecting the microanatomy of the lateral geniculate body of adult wistar rats. It is recommended for further studies aimed at corroborating these observations.
Keywords: Histological Effects. Nutmeg. Lateral Geniculate Body. Wistar rats.
RESUMEN: EFECTOS HISTOLÓGICOS DEL CONSUMO CRÓNICO DE NUEZ MOSCADA EN EL CUERPO GENICULADO LATERAL DE RATAS WISTAR ADULTAS.
Hemos estudiado cuidadosamente los efectos del consumo crónico de nuez moscada, comúnmente utilizada para condimentar platos diversos o añadir a tés y bebidas sin alcohol mezclada con leche y alcohol, sobre el cuerpo geniculado lateral de ratas Wistar adultas.
Un lote de ratas de ambos sexos, con un peso promedio de 200g fueron asignados al azar en grupos de tratamiento y control. Las ratas del grupo de tratamiento (n = 8) recibieron 2g de nuez moscada bien mezclada con los alimentos, durante treinta y dos días. El grupo control (n = 8) recibió la misma cantidad de alimentos al día sin nuez moscada añadida durante los mismos treinta y dos días. El pienso molido se obtuvo de Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria y a las ratas se les dio agua abundante. Los animales se sacrificaron el día treinta y tres del experimento. El cuerpo geniculado lateral se disecó con cuidado y fué rápidamente fijado en formol salino al 10% para estudio histológico.
Los resultados indican que las ratas en el grupo tratado, mostraron algunos cambios celulares degenerativos como dispersión celular, picnósis nuclear con algunos cambios microquísticos, edema y vacuolizaciones en el estroma del cuerpo geniculado lateral de los animales tratados, en comparación con el grupo control.
El consumo crónico de nuez moscada por tanto, puede tener un efecto adverso sobre la sensibilidad visual al afectar la microanatomía del cuerpo geniculado lateral de ratas Wistar adultas. Se recomiendan estudios adicionales encaminados a corroborar estas observaciones.
Palabras clave: Efectos histológicos. Nuez Moscada. Cuerpo geniculado lateral. Ratas Wistar.
INTRODUCTION
The nutmeg plant, Myristica fragrans Houtt, is a member of the small primitive family called Myristicaceae, taxonomically placed between the Annonaceae and Lauraceae1. At present, Myristicaceae is considered as a member of Magnotiales or its taxonomical equivalents2-3. Nutmeg has long been known for its psychoactive properties, producing anxiety/fear, hallucination, from as early as 16th century writings to current internet based site4-6.
Nutmegs are widely accepted as flavoring agents, are used in higher doses for their aphrodisiac and psychoactive properties in male rat7-8. Nutmeg and its Oleoresin are used in the preparation of meat products, soaps, sauces, baked foods, confectioneries, puddings, seasoning of meat and vegetables, to flavour milk dishes and punches. Powdered nutmeg is rarely administered alone, but enters into the composition of numerous medicines as aromatic adjuncts. Medicinally, nutmeg is known for its stimulative and carminative properties9-10. In pregnancy and lactation, traditionally nutmeg has been used as an abortive agent. Although this use has been largely discounted, it remains a persistent cause of nutmeg intoxication in women11.
The active ingredient in nutmeg is called Myristicine and is a naturally occurring insecticide and acaricide with possible neurotoxic effects on dopaminergic neurons and a monoamine oxide12-13. Cytotoxic and apoptotic effects of Myristicine have been reported such that cell viability was reduced by exposure to Myristicine in a dose dependent manner13.
The superior colliculus and lateral geniculate body constitutes the intracranial visual relay centres. The lateral geniculate body in mammals is considered as part of the thalamic nuclei for processing visual information14. In rats the lateral geniculate body receives input from the geniculate leaflet, which participates in the regulation of circadian function through its projection to the circadian pacemaker of the hypothalamus15. Cortical structures such as the medial and lateral geniculate bodies, inferior and superior colliculi have higher glucose utilization than other structures16. There is a correlation between functional activity and metabolic rate such as in the visual and auditory system16.
Since the neurons of the central nervous system are affected by nutmeg, it is relevant to investigate its effect on the lateral geniculate body since there is no actual dosage used specification by various food vendors in Nigeria. It is probable that the adverse cytotoxic and apoptotic effects of myristicine reported may be due to direct effect of nutmeg on the lateral geniculate body. This present study is to investigate some histological effects of chronic consumption of nutmeg on the lateral geniculate body of adult wistar rats.
MATERIALS AND METHODS
ANIMALS: Sixteen adult wistar rats of both sexes with average weight of 200g were randomly assigned into two groups: A and B of (n=8) in each group. Group A served as treatment group (n=8) while group B (n=8) served as the control. The rats were obtained and maintained in the Animal Holding of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Edo State, Nigeria. The animals were fed with grower's mash obtained from Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria and given water liberally. The Nutmeg seeds were obtained from Oba Market, Benin City, Edo State. They were dried and graded into powder at the Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, Benin City.
NUTMEG ADMINISTRATION: The rats in the treatment group (A) were given 2g of Nutmeg thoroughly mixed with the growers mash respectively on a daily basis for thirty-two days. The control group (B) received equal amount of feeds without Nutmeg added for the same period of thirty- two days. The rats were sacrificed by cervical dislocation on the thirty- three day of the experiment. The skulls were opened using bone forceps to expose the brain of the rats. The lateral geniculate body was quickly dissected out and fixed in 10% formal saline for routine histological techniques.
HISTOLOGICAL STUDY: The tissues were dehydrated in an ascending grade of ethanol, cleared in xylene and embedded in paraffin wax. Serial sections of 7 microns thick were obtained using a rotatory microtome. The deparaffused sections were stained routinely with haematoxyline and eosin17.
Photomicrographs of the desired results were obtained using research photographic microscope in the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria.
RESULTS
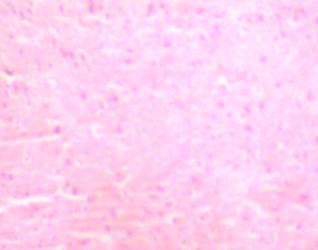
The desired sections of the lateral geniculate body (LGB) from the control animals showed normal histological features with the neurons appearing distinct and of various sizes. The neuron and glial cells appeared normal and no vacuolation in the stroma of the sections (figures 1 - 2).
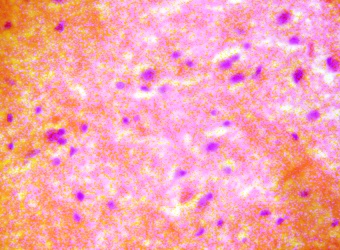
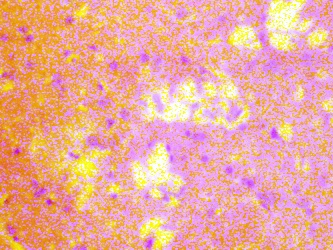
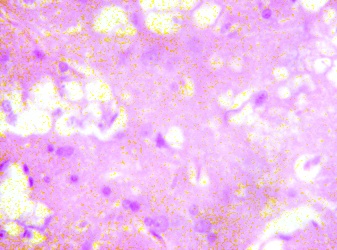
The lateral geniculate body (LGB) of the treated groups revealed some cellular degenerative changes like sparse cellular population, pyknotic nuclei with some microcystic changes, edema and vacuolations in the stroma of the treated lateral geniculate body (LGB) as compared to that of the control group (figures 3 - 4).
DISCUSSION
The results (H&E) of this experiment revealed some cellular degenerative changes like sparse cellular population, pyknotic nuclei with some microcystic changes, edema and vacuolations in the stroma of the treated lateral geniculate body as compared to the control section of the lateral geniculate body in adult wistar rats. Neuronal degeneration has been reported to result in cell death, which is of two types, namely apoptotic and necrotic cell death. These two types differ morphologically and biochemically18. Pathological or accidental cell death is regarded as necrotic and could result from extrinsic insults to the cell such as osmotic, thermal, toxic and traumatic effects19. It was reported that cell death in response to neurotoxins might trigger an apoptotic death pathway within brain cells20. Cell death in response to neurotoxins occurs as a controlled event involving a genetic programme in which caspase enzymes are activated20.
The process of cellular necrosis involves disruption of the membranes structural and functional integrity. Cellular necrosis is not induced by stimuli intrinsic to the cells as in programmed cell death (PCD), but by an abrupt environmental perturbation and departure from the normal physiological conditions21.
There is the need to further investigate the actual mechanism by which nutmeg induced neuronal degeneration in the lateral geniculate body of adult wistar rats in this study.
Extensive cell death in the central nervous system is present in all neurodegenerative diseases20. The type of nerve cell loss and the particular part of the brain affected dictate the symptoms associated with an individual disease20. In this study nutmeg may have acted as toxin to the cells of the lateral geniculate body, affecting their cellular integrity and causing defect in membrane permeability and cell volume homeostasis.
In cellular necrosis, the rate of progression depends on the severity of the environmental insults. The greater the severity of the insults the more rapid the progression of neuronal injury22. The principle holds true for toxicological insult to the brain and other organs21. The prime candidates for inducing the massive cell destruction observed in neurodegeneration are neurotoxins20. These may be substances present in small amounts in the environment, or even naturally occurring chemicals such as glutamate used by the brain as transmitter's substances20. The latter when present at a critical level can be toxic to the brain cells they normally excite20. It could be inferred from this results that prolonged and high dose of nutmeg resulted in increased toxic effects on the lateral geniculate body. The decrease in cellular population observed in this study may have been as a result of cell death caused by the toxic effect of nutmeg. In the same way, it has been reported that chronic administration of chloroquine resulted in the cellular degenerative changes, sparse cellular population and vacuolation appearing in the stroma with some autophagic vacuoles in the inferior colliculus and medial geniculate body of adult Wistar rats23-24.
The vacuolations observed in the stroma of the treated lateral geniculate body in this experiment may be due to nutmeg interference, since it has been reported that myristicine obtained from the nutmeg may have a cytotoxic and apotoxic effects on the body. The sparse cellular population observed in this experiment may be due to the adverse effects of nutmeg on the cells of the lateral geniculate body of the adult wistar rats.
The microcystic changes and edema observed in the stroma of the lateral geniculate body in this experiment may be due to nutmeg interference, since it is known to cross blood brain barrier and thus getting access to the cells of the brain. The results observed in this experiment may be due to the adverse effects of nutmeg on the lateral geniculate body. Since the neurons of the central nervous system is affected by nutmeg, it is probable that the results obtain in this experiment may have been due to the neurotoxic effect of nutmeg on the neuronal cells of the lateral geniculate body of treated adult wistar rats.
Ischemic or pharmacologic disruption of cellular transporters can cause swelling of the brain parenchyma. Under such conditions, there is a net shift of water from the extracellular space to the interior of the brain cells25. Cytotoxic edema usually involves intracellular swelling of glial, endothelia and neurons25. The microcystic changes and edema in the stroma of the treated lateral geniculate body reported in this experiment might be due to neurotoxic effect of nutmeg on the lateral geniculate body of adult wistar rats. Regulation of brain water content and therefore of the volume is critical for maintaining the intracranial pressure within tolerable limits25. In this study nutmeg could have acted as toxins to the cell of the lateral geniculate body thus affecting their cellular integrity and causing a defect in membrane permeability and cell volume homeostasis.
As brain tissue swells or shrinks as seen in this study, the activity of the cellular transporters is approximately modified by the up or down regulations as earlier reported in the case of hyponatremia or hypernatremia25. Ischemia or pharmacologic disruption of cellular transporters can cause swelling of parenchyma of the brain and that of the lateral geniculate body. The pharmacologic disruption of the lateral geniculate body caused by nutmeg is a cardinal feature of the results of this experiment. Though there are many different causes of cell swelling, including drug poisoning, water intoxication, hypoxia, and acute hyponatremia25. Under such conditions there is a net shift of water from the extracellular space to the interior of the brain cells25. The results observed in this experiment, usually involves intracellular swellings of glial, endothelia and neurons25. Brains swelling attendant to severe cytotoxic edema may lead to marked reduction in the size of the ventricular system and basal cisterns25.
Since the neurons of the central nervous system is affected by nutmeg, it is probable that the results obtain in this experiment may have been due to the neurotoxic effect of nutmeg on the neuronal cells of the lateral geniculate body of adult wistar rats.
CONCLUSION AND RECOMMENDATION
This study revealed that high doses and long term consumption of nutmeg causes some cellular degenerative changes like sparse cellular population, pyknotic nuclei with some microcystic changes, edema and vacuolations in the stroma of the treated lateral geniculate body as compared to the control section of the lateral geniculate body in adult wistar rats. These results may probably affect the visual sensibility functions of the lateral geniculate body in the adult wistar rats.
REFERENCES
-
1. Joseph J. The Nutmeg, its Botany, Agronomy, Production, Composition and Uses. 1980; 2: 61-67
2. Cronquist A. An integrated system of classification of flowering plants. Columbia University press. New York. 1983
3. Dahlgren R. General aspect of angiosperm evolution and macro systematics. Nordic Journal botany. 1983; 3:119 -149
4. Brenner N, Frank OS, Knight E. Chronic Nutmeg Psychosis. 1993; 86:179-180
5. Kelly BD, Gavin BE, Clarke M, Lane A, Larkin C. Nutmeg and Psychosis. Schizophr Res. 2003; 60: 85 - 96.
6. Forrester MB. Nutmeg Intoxication in Texas, 1998 - 2004. Hum Exp. Toxicol. 2005; 24: 563-566
7. Tajuddin S, Ahmad S, Latif A, Qasmi IA. Aphodisiac Activity of 50% Ethanol extract of myristica fragrans Houtt (Nutmeg) and Syzygium Aromaticum (L) Merr andPerry. (Clove) in male mice. A Comparative study. BMC Complement Altern. Med. 2003; 3:6
8. Tajuddin S, Ahmad S, Latif A, Qasmi IA, Amin KM. An Experimental Study Of Sexual Function Improving Effect of Myristica fragrans. Houtt. BMC Complement Altern Med. 2005; 5:16
9. Madsen HL, Bertelsen G. Spices as antioxidants. Trends Food Sci Technol. 1996; 6: 271- 277
10. Lagouri V, Boskou D. Screening for Antioxidant Activity of Essential Oils obtained from spices. In food flavors: Generation, analysis and process influence ( ed Charalambous G), Amsterdam, Elsevier. 1995; pp. 869-879.
11. De Milto L, Frey RJ. Nutmeg. In: Longe J.L, Project editor, Gale Encyclopedia of Alternative Medicine. Vol.3 2nd ed. Detroit, ML. Thomson Gale. 2005; 1461-1463
12. Truitt EB, Duritz G, Ebersberger EM. Evidence of Mondamine Oxidase Inhibition by Myristicin and Nutmeg.Proc Soc Exp Biol Med. 1963; 112: 647-650
13. Lee BK, Kim JH, Jung JW. Myristicin-induced Neurotoxicity in Human neuroblastoma SK-N-SH Cells. Toxicol Lett. 2005; 157: 49- 56
14. Altman AS, Bayer CS. Time of Origin of neurons of rat superior colliculus in relation to other components of the visual and visuomotor pathways. Experimental Brain Research.1981; 42: 424-434
15. Moore RY, Card JP. Intergeniculate leaflet: An anatomically and Functionally Distinct subdivision of the lateral geniculate complex. J. Comparative Neurology. 1984; 344: 403-444
16. Siesjo BK. Utilization of substrates by brain tissues. Brain energy metabolism. John Wiley and Sons, USA. 1978; 101-130.
17. Drury RAB, Wallington EA, Cameron R. Carleton's Histological Techniques: 4th ed., Oxford University Press NY. U.S.A. 1967; 279-280.
18. Wyllie AH. Glucocorticoid-induced thymocyte apoptosis in associated and endogenous endonuclease activation. Nature: London. 1980; 284:555-556.
19. Farber JL, Chein KR, Mittnacht S. The pathogenesis of Irreversible cell injury in ischemia. Am J Pathol 1981; 102:271-281
20. Waters CM. Glutamate induced apoptosis of striatal cells in rodent model for Parkinsonism. Neuroscience. 1994; 63:1-5
21. Martins LJ, Al-Abdulla NA, Kirsh JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischaemia and target Deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 1978; 46(4): 281-309.
22. Ito U, Spatz M, Walker JT Jr, Klatzo I. Experimental cerebral ischemia in Mongolian Gerbils. I. Light microscopic observations. Acta Neuropathol. 1975;32:209-223.
23. Adjene JO, Adenowo TK. Histological studies of the effect of chronic administration of Chloroquine on the inferior colliculus of adult Wistar rat JMBR. 2005; 4: 83-87.
24. Adjene JO, Caxton-Martins AE. Some histological effects of chronic administration of chloroquine on the medial geniculate body of adult wistar rat. Afr J Med Med Sci. 2006;35:131-135.
25. Johanson CE. Effects of Fluid in Balances. Neuroscience in Medicine. P. Michael Conn, J.B. Lippincott Company, 1995; pp.187-189.
Correspondence:
J.O. Adjene
Department of Anatomy, School of Basic Medical Sciences
University of Benin
Edo State, Nigeria
E-Mail: joadjene @ yahoo.com