Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
MORPHOMETRIC EFFECTS OF COLA NITIDA EXTRACT ON THE STOMACH OF ADULT MALE WISTAR RATS
Ojo, Gideon B.*, Caxton-Martins, Ezekiel A.**, Odukoya, Samson O. A.*
* Department of Anatomy, Faculty of Basic Medical Sciences,
College of Health Sciences, Bowen University, Iwo.
**Department of Anatomy and Cell Biology,
Obafemi Awolowo University, Ile-Ife,
Nigeria.
ojogbfavour @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2010;2:18-24.
Comment of the reviewer reviewer Prof. Pilar Muńiz Rodríguez PhD. Biochemistry and Molecular Biology. Sciences Faculty. Universidad de Burgos. Espańa
Comment of the reviewer reviewer Prof. Maxim V Trushin PhD. Professor at Department of Genetics of Kazan State University. Kazan. Russia
ABSTRACT
Cola nut was investigated for possible harmful effect on the morphology of the stomach, considering its wide consumption and documented antioxidant properties.
Twenty-five Adult male Wistar rats with average weight of 167.6 g and randomly divided into five groups A, B, C, D and E each containing five animals. Care of the animal according to the Rules and Guidelines of the Animal Right Committee of the Obafemi Awolowo University, Ile-Ife, Nigeria was adopted. The rats in group A (control) were given distilled water while animals in experimental groups B, C, D and E were each given 600 mg/kg body weight of crude extract of Cola nitida by oral intubation for consecutive three, five, seven and nine days respectively and sacrificed. The stomach was excised, quickly fixed in 10% formal saline and processed histologically, using routine haematoxylin and eosin (H and E) stain. The stained sections were subjected to morphometrics analysis at a magnification of sign 40 using the eye piece micrometer procedure.
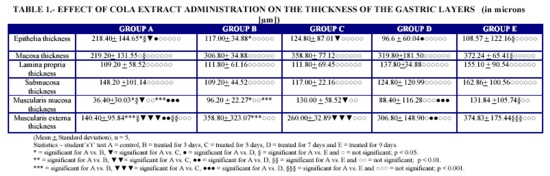
The result revealed a significant reduction in the epithelia thickness of the experimental animals, (Groups A=218.40 µm ± 144.61 vs. B=117.00 µm ± 34.88, C=124.80 µm ± 87.01, D=96.60 µm ± 60.04 and E=108.57 µm ± 122.16) (t=3.04, 2.48, 3.57 and 2.58 respectively, p < 0.05).
The thickness of the lamina propria and submucosa was not significant in all cases of the experimental when compared with the control animals (Groups A=109.20 µm ± 58.52 vs. B=111.80 µm ± 61.18, C=111.80 µm ± 69.45, D=137.80 µm ± 34.88 and E=155.10 µm ± 90.54) (t=0.14, 0.13, 1.88, 1.90 respectively, p>0.05) and (A=148.20 µm ± 50.56 vs. B=109.20 µm ± 22.27, C=117.00 µm ± 11.07, D=124.80 µm ± 71.67, E=162.86 µm ± 112.35) (t=1.58, 1.35, 0.60, and 0.46 respectively, p<0.05).
The thickness of the muscularis mucosa and muscularis externa were significantly increased by the extract, (Groups A=140.40 µm ± 95.84 vs. B=358.80 µm ± 323.07, C=260.00 µm ± 32.89, D=306.80 µm ± 148.90, E=374.83 µm ± 175.44) (t=7.16, 6.36, 3.83, and 2.89 respectively, p<0.05) and (140.4 µm ± 47.94 vs. B=358.80 µm ±161.53, C=260.00 µm ± 16.44, D=306.80 µm ± 74.44, E=374.83 µm ± 87.72) (t=2.90, 5.22, 4.20, and 5.22 respectively, p<0.05).
It is therefore evident that the consumption of cola nut leads to a reduction in the epithelia thickness and a significant increase in the thicknesses of muscularis mucosa and the muscularis externa and however this is as a result of increase in the secretomotor activity of the stomach when cola nut is ingested.
KEYWORDS: Cola nut. Glands. Cola extract. Secretions. Morphometric
RESUMEN: EFECTOS MORFOMÉTRICOS DEL EXTRACTO DE COLA NITIDA SOBRE EL ESTÓMAGO DE RATAS WISTAR, MACHO ADULTAS
La nuez de cola fue investigada por posible efecto nocivo sobre la morfología del estómago, teniendo en cuenta su amplio consumo y sus documentadas propiedades antioxidantes.
Veinticinco ratas Wistar machos adultas con peso promedio de 167,6 gramos se dividieron aleatoriamente en cinco grupos: A, B, C, D y E cada uno con cinco animales. El cuidado de los animales fue de acuerdo con las Normas y Directrices de la Comisión Animal Derecho de la Universidad Obafemi Awolowo, Ile-Ife, Nigeria. Las ratas del grupo A (control) recibieron agua destilada mientras que los animales en los grupos experimentales B, C, D y E recibieron cada una 600 mg / kg de peso corporal del extracto crudo de Cola nitida mediante intubación oral por períodos consecutivos de tres, cinco, siete y nueve días, respectivamente, al cabo de los cuales fueron sacrificadas. El estómago fue extirpado quirúrgicamente y fijado inmediatamente en solución de formol salino al 10% y procesado histológicamente, y tenido con hematoxilina y eosina (H y E). Los cortes teńidos fueron sometidos a análisis de morfometría, con un aumento de 40 utilizando un micrómetro ocular.
El resultado reveló una reducción significativa en el espesor del epitelio de los animales de experimentación, (Grupos A = 218.40 µm ± 144.61 vs. B=117.00 µm ± 34.88, C=124.80 µm ± 87.01, D=96.60 µm ± 60.04 and E=108.57 µm ± 122.16) (t=3.04, 2.48, 3.57 and 2.58 respectivamente, p < 0.05).
El espesor de la lámina propia y submucosa no fue significativa en todos los casos de cuando se comparan con los animales control (Grupos A=109.20 µm ± 58.52 vs. B=111.80 µm ± 61.18, C=111.80 µm ± 69.45, D=137.80 µm ± 34.88 and E=155.10 µm ± 90.54) (t=0.14, 0.13, 1.88, 1.90 respectively, p>0.05) y (A=148.20 µm ± 50.56 vs. B=109.20 µm ± 22.27, C=117.00 µm ± 11.07, D=124.80 µm ± 71.67, E=162.86 µm ± 112.35) (t=1.58, 1.35, 0.60, and 0.46 respectivamente, p<0.05).
El espesor de la muscularis mucosa y muscular externa se incrementaron de manera significativa por el extracto, (Grupos A=140.40 µm ± 95.84 vs. B=358.80 µm ± 323.07, C=260.00 µm ± 32.89, D=306.80 µm ± 148.90, E=374.83 µm ± 175.44) (t=7.16, 6.36, 3.83, and 2.89 respectively, p<0.05) y (140.4 µm ± 47.94 vs. B=358.80 µm ±161.53, C=260.00 µm ± 16.44, D=306.80 µm ± 74.44, E=374.83 µm ± 87.72) (t=2.90, 5.22, 4.20, and 5.22 respectivamente, p<0.05).
Por tanto, es evidente que el consumo de nuez de cola lleva a una reducción en el espesor de los epitelios y un aumento significativo en el grosor de la mucosa y la muscular muscular externa. Esto se produce como resultado del incremento de la actividad secretomotora del estómago cuando se ingiere la nuez de cola.
PALABRAS CLAVE: Nuez de Cola. Glándulas. Extractos de Cola. Secreciones. Morfometría.
INTRODUCTION
In Nigeria it is generally acknowledged that the Yorubas grow the Cola nut; the Hausas eat it, while the Igbos celebrates it. In Igbo land it is a common saying that 'he who brings cola brings life', it is a sign and gesture of friendship, this shows that Cola nut cut across all the major tribes in Nigeria and it is therefore a plant of importance1-4. The seeds of cola nut are considered a symbol of hospitality in Africa they are taken as a stimulant, antioxidant and masticatory, but it was reported to aggravate gastric and duodenal ulcer by increasing the level of gastric acid produced suggesting effect on the stomach morphology. This study investigated the effects of cola nut ingestion on the stomach of adult male Wistar rats as it affect the morphometrics of the different histologic layers of the stomach.
There are four layers in the stomach according to the general plan of the gastrointestinal tract5-7, which are as follows:
I Mucosa: The surface of the stomach is lined by simple columnar epithelium whose cells are made up of surface mucous cells. There are numerous invaginations of the surface epithelium, which extends into the lamina propria. The surface mucous cells produce a cloudy, viscous and alkaline mucous that forms a thick gel-like coat that adheres to the surface epithelium and protect it from abrasion and the acid content of the stomach8-12. The mucosa is thrown into longitudinal folds also called gastric folds or ruggae, which disappear when the stomach is fully distended.
Glands are present in the lamina propria which separates the gastric pits that are parallel to each other; the gastric pits occupy approximately 25% of the mucosa, the glands in the lamina propria empty into the bases of the gastric pots: the stomach is divided into three histological regions based on the nature of the glands:
1. Cardiac region is a narrow band near the opening of the esophagus, which contains cardiac glands. The glands are branched simple tubular glands and are composed almost entirely of mucus secreting cells with few odd enteroendocrine cells present and few secretory cell characteristic for the fundic regions may be present10.
2. Fundic region: this constitutes the majority of the stomach. The glands in this region are oriented more or less perpendicular to the surface epithelium, they are known as gastric, principal or fundic glands and extend all the way to the muscularis mucosae. About three to seven glands open into the base of each gastric pit. Each gland consists of three parts: a deep body or base; an intermediary fairly long and narrow neck and an upper isthmus. At their base the gland may divide into two or three branches which become slightly coiled. In the fundic region, almost all the entire lamina propria is occupied by glands. The lumina of the glands are usually not identifiable and they usually appear more like cords of cells. The only typical lamina propria can be seen in areas between the fovealae and around the bases of the glands. The following cell types can be seen in the glands of the fundic region:
a. Mucous neck cells: these are located between the parietal cells in the neck region of the gland, they secrete soluble mucous only under vagal stimulation. They are shorter than the surface mucous cells
b. Parietal or oxyntic cells: these occur predominantly in the neck of the gland interspersed among the mucous neck cells, they are situated deeper, between and below chief cells in lower part of the gland. They secrete hydrochloric acid (HCl) of the gastric juice and intrinsic factor. They are intensely eosinophilic due to the amount of membrane comprising an extensive intracellular canalicular system and numerous mitochondria. The HCl secretion is stimulated mainly by gastrin; they activate pespinogen and effectively sterilize the stomach. The intrinsic factor is a glycoprotein that binds vitamin B12, they are necessary for resorption of vitamin B12 which is essential for maturation of red blood cells9,10,12
c. Chief or zymogenic cells: they are the most numerous of all the cell types and are located primarily in the body of the glands and are protein secreting cells. Their basophilia stems from their abundance of rough endoplasmic reticulum. They secrete pepsinogen which is a precursor of the proteolytic enzyme pepsin to which it is being converted upon contact with gastric acid, the optimum pH of pepsin is 2, this enzyme is able, to break collagen.
d. Entero endocrine or Neuroendocrine cells: These cells are prevalent near the base but can be found anywhere in the glands. They are not readily identifiable in histological preparations but show up with various silver stains and hence were also known as argentaffin and argyrophilic cells, other names include enterochromaffin and APUD cells (Amine precursor uptake and decarboxylation cells). Enteroendocrine cells secrete their product into the lamina propria where it is taken up by blood vessels. Thus, stains that reveal their granites show them to be at the basal, rather than the lumina. The major secretory product of the enteroendocrine cells of the stomach is gastrin secreted by the G cells which stimulate the production of HCl; somatostatin secreted by D cells which inhibit G cells and thereby HCl production; other type of products are VIP (vasoactive intestinal peptide) secreted by D cells, glucagon, serotonin and substance P.
e. Undifferentiated cells or stem cells: These cells are found in the neck region and give rise to all the other cell types. They are low columnar cells and are few in number, their presence is only revealed in preparations treated with tritiated thymidine, they travel upwards to replace surface mucous cells whose life span is 3-5 days and downward to replace parietal, chief and enteroendocrine cells whose life span is about a year 9,10,12-13.
3. Pyloric region is the part of the stomach proximal to the pyloric sphincter and contains pyloric glands. They are short, more coiled, have branched tubular glands with a wide lumen. Their cells secrete mucous and are similar in appearance to the surface mucous cells also present are the enteroendocrine cells mainly G cells which are more frequent than in the principal glands a few parietal cells may be present but chief cells are usually absent10.
The lamina propria is formed by a very cell-rich loose connective tissue made up of fibroblasts, lymphocytes, plasma cells, macrophages, eosinophilic leucocytes and mast cells (10).
The muscularis mucosa of the stomach consists of an inner circular and outer longitudinal layer. In some areas, a third usually circular layer is present. Strands of smooth muscle extend from the inner layer into the lamina propria; they are thought to be involved in facilitating the outflow from the gastric glands12.
II. Submucosa: This is made up of smooth muscle which supports the mucosa consisting of dense connective tissue which contains large blood vessels, lymph vessels and nerves plexus of Meissner10.
III. Muscularis externa or muscularis propria: This consists of three fairly indistinct layers; an inner oblique middle circular and outer longitudinal layer (14). The layers are somewhat randomly oriented and some are absent or poorly developed in some areas. The muscularis mixes the chyme and expels it into the small intestine, the muscularis contains Meissner's plexus, but they are located between the circular and longitudinal fibers of the muscularis externa and concentrated in the submucosa, myenteric plexus, superior mesenteric plexus and Auerbach's plexus10.
IV. Adventitia or Serosa: The stomach is covered by a serosa which is continuous with the peritoneum of the body via the omentum, it consist of blood vessels, nerves and adipose tissues 10,12,15
MATERIALS AND METHODS
Care and Management of The Animals: The animals were reared in the Animal House of the Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ile - Ife, Nigeria. They were kept in metabolic cages and fed commercially available rat chow bought from Guinea Feeds Limited Ewu, Edo State, and water was given freely. They were left to acclimatize for two weeks. The weight of the animals was taken once a week. The experiment involved twenty five presumably healthy adult male Wistar rats with average weight of 167.6 g and randomly divided into five groups A, B, C, D and E each containing five animals. Care of the animal according to the Rules and Guidelines of the Animal Right Committee of the Obafemi Awolowo University, Ile-Ife, Nigeria was adopted.
Preparation of Cola nitida Extract: Fresh kola nut seeds were purchased from local market in Ile - Ife, Nigeria. The seeds weighed 100 g and were cut into pieces and dried; the dried seeds were ground into powder, using the electric grinding machine in the Department of Pharmacology, Obafemi Awolowo University Ile - Ife, Nigeria and extraction done using 70% ethanol in a Soxhlet extraction, the powder which weighed 2.35 g was poured into the solvent for three days. The extract was filtered using glass funnel and cotton wool plug, the residue were discarded and the filtrate kept and taken to the Science Central Laboratory, Obafemi Awolowo University, Ile - Ife, Nigeria where it was concentrated using rotary evaporator and freeze-dried into a solid mass. 0.60 g of the extract was weighed out and dissolved in 1 ml of distilled water to give 600 mg/ml/kg 16-17 of ethanolic extract (Cola nitida extract).
Administration Of Extract And Animal Sacrifice: Animals in group A (control) were given distilled water, animals in Groups B, C, D and E which serve as experimental groups given 600 mg/kg body weight of aqueous extract of Cola nitida via oral route18 using oropharyngeal tube. The extract was administered for three, five, seven and nine days respectively. All the animals were sacrificed by cervical dislocation. Ventral abdominal incision was carried out on the animals and the organ of choice; the stomach was excised, stomach tissues for histological analysis were fixed in 10% formal saline, the area of interest in the stomach is the corpus (body) where most of the digestive action of the stomach takes place.
Morphometric Analysis: Morphometric analysis was carried out on the epithelial thickness, mucosa thickness, lamina propria thickness, muscularis mucosa thickness, submucosa thickness and muscularis externa thickness.
The microscope was calibrated by the Eyepiece Micrometer procedure19-21
RESULTS
It was noted from table 1 that the effect of cola extract administration on epithelia thickness was significant when the control was compared with the test groups (p < 0.05). It was noted from table that the effect of cola extract administration on mucosa thickness when the control was compared with the test groups was only significant at day nine (p < 0.05).
It was noted from table 1 that the effect of cola extract administration on the thickness of size of the lamina propria when the control was compared with test groups was not significant (p > 0.05).It was noted from the table that the effect of cola extract administration on the submucosa thickness when the control animal was compared with the test groups was found to be statistically insignificant (p > 0.05).
It was noted from table 1 above that cola extract have a significant effect on the thickness of the muscularis mucosa for three, five and nine days of administration respectively, it was however found to be insignificant for the seventh day of administration (p > 0.05). It was noted from the table above that cola extract have a significant effect on the thickness of the muscularis externa for three, five, seven and nine days of administration when compared with the control group (p < 0.05). It was however found insignificant for the seventh day of administration (p > 0.05).
It was noted from the table that the effect of cola extract on the thickness of the epithelium was statistically insignificant for all groups (p > 0.01). It was also found to be insignificant for all groups on the thickness of the mucosa of experimental animals for three, five and seven day when the control was compared with the experimental. (p > 0.01). It was however significant for animal treated for nine day (at p < 0.01).
It was also noted from the table that the effect of cola extract administration on the thickness of the lamina propria when the control was compared with test groups was not significant (p > 0.01). It was noted from the table that the effect of cola extract administration on the submucosa thickness when the control animal was compared with the test groups was found to be statistically insignificant (p > 0.01)
Cola extract was also found to have statistically insignificant effect on the thickness of the muscularis mucosa when the control was compared with the experimental groups (p < 0.01).
Cola extract administration was also found to have a highly significant effect on the thickness of the muscularis externa for animals treated for three, five, seven and nine days when compared with the control (p > 0.01).
It was noted from table that the effect of cola extract on the epithelia thickness was found to be statistically insignificant for all groups (p > 0.001). It was also not significant on the mucosa thickness for all groups (p > 0.001).
The effect on the lamina propria thickness, submucosa thickness and muscularis externa thickness also found to be statistically insignificant for all groups (p > 0.001). The effect on the thickness of the muscularis mucosa was however found to be very highly significant for animals treated for 3 and 5 days (p < 0.001) and not significant for animal treated for seven and nine days (p > 0.001).
DISCUSSION
The results of the epithelia thickness of the treated animals with the control using students't' test was found to be significantly reduced (p <0.05). This gives more credence to earlier reports that the use of kola nut and other plant extract leads to necrotized, eroded and degraded epithelia lining of stomach. This was as a result of ulceration of the epithelia lining of the stomach18-22. The results of the lamina propria thickness, submucosa thickness of treated animals compared with control using students 't' test was found to be insignificant (p> 0.05) this confirms earlier report on the effect of cola nut intake on the lamina propria and submucosa 24,27,28.
The results of the muscularis mucosa thickness and serosa thickness of treated animals compared with control using students 't' test was found to be significantly increased (p <0.05 and p <0.01) this gives more credence to earlier reports that kola nut extract ingestion significantly induce gastric acid secretion by acting upon circular fibres of the stomach mucosa and so increasing secretion leads to increasing release of the gastric secretions into the gastric pits22,23.
CONCLUSIONS:
The results obtained in this study following the administration of 600 mg/kg body weight of ethanolic extract of cola nitida to adult male Wistar rat causes degradation of the epithelia lining of the stomach. This is as a result of the increase in the number of acid secreting cells and an increase in the activity of the acid secreting cells leads to the wearing away of the surface epithelium which accompany this activity.
Morphometric studies revealed a reduction in thickness of the epithelia lining, increase in thickness of mucosa and muscularis which leads to increase in secretomotor activity of the stomach. It is therefore concluded that kola nut that is taken by people of all ages and cadres should be taken with moderation as it has its own side effect putting to rest the erroneous view that kola nut ingestion have no side effect.
REFERENCES
-
1.- Sundstrom L. The cola nut functions in West African Social Life. Studia Ethnographica Upsaliensis (Stockholm: Almqvist and Wiksell) 1966;28:135-149.
2.- Hauenstein A. La noix de cola: costumes' et rites de quelques ethaies de cote d'Ivoire. Anthropos 1971;69:457-493.
3.- Lovejoy PE. Kola in the History of West Africa. Cahier d'etudes africaines. 1980; XX (1-2): 77-78
4.- Trindall R. Ethnobotanical Leaflets: The Culture of Cola: Social and Economic Aspects of a West African Domesticate. Carbondale: Southern Illinois University Herbarium 1997.
5.- Hellberg H, Bjerkĺs I. The anatomy of the oesophagus, stomach and intestine in common wolffish (Anarhichas lupus L.): a basis for diagnostic work and research. Acta Vet Scand. 2000;41:283-297.
6.- Arellano JM, Storch V, Sarasquete C. Histological and histochemical observations in the stomach of the Senegal sole, Solea senegalensis. Histol Histopathol. 2001;16: 511-521.
7.- Suíçmez M, Ulus E. A study of the anatomy, histology and ultrastructure of the digestive tract of Orthrias angorae Steindachner, 1897. Folia Biol (Krakow). 2005;53(1-2):95-100.
8.- Filipe MI. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979;2:195-216.
9.- Fawcett DW. A Textbook of Histology. Twelfth edition. Ed. Chapman and Hall. New York, 1994.
10.- Sternberg, S. Stephen (editor). Histology for Pathologists 2nd Edition: Lippincott-Raven Publishers, Philadelphia, 1977.
11.- Berman I. Colour Atlas of Basic Histology, Second edition. Appleton and Lange, Stanford, Connecticut, 1998.
12.- Young B, Health JW. Wheater's. Functional Histology: A text and Colour Atlas 4th edition. Churchill Livingstone. Harcourt Publishers, 2000.
13.- Matsuyama M, Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil and chief cells in stomach grafts. Science. 1970;169: 385-387.
14.- Reitel CW, Travill AA. Structure and Carbohydrate histochemistry of the stomach in eight species of Teleos. Journal of Morpholology. 1978:158: 155-167.
15.- Leeson CR, Lesson TS, Paparo AA. Textbook of histology. WB Saunders. Philadelphia 1985
16.- Ettarh RR, Okoosi, SA, Eteng MU. The influence of kolanut (Cola Nitida) on exploratory behaviour in rats. Pharm Biol. 2000;38:281-283.
17.- Chukwu LO, Odiete WO, Briggs LS. Basal metabolic regulatory responses and rhythmic activity of mammalian hearts to aqueous kola nut extracts. Afr. J. Biotechnol. 2006;5:484-486
18.- Monosson E. (Ed). Environmental Health, Ecotoxicology and Risk Assessment Absorption of Toxicants in Encyclopedia of Earth. National Library of Medicine. Washington BC 2007.
19.- World Health Organization. Calibrating the Microscope. In Basic Laboratory Methods in Medical Parasitological. 1991
20.- Baker FJ, Silverton RE, Kilshaw D, Shannon S, Couthine DL, Eggleston S, Mackenzie JC. Laboratory Technology 6th edition Butter Worths & Co. (Publishers) Ltd, 1985.
21.- Ofusori DA, Caxton-Martins EA, Adenowo TK, Ojo GB, Falana BA, Komolafe AO, Ayoka AO, Adeeyo AO, Oluyemi KA. Morphometric study of the stomach of African pangolin(Manis tricuspis). Sci. Res. Essays. 2007;2:465-467.
22.- Ibu JO, Iyama AC, Ijije CT, Ishmael D, Ibeshim M, Nwokediuko S. The effect of cola acuminata and cola nitida on gastric acid secretion. Scand J Gastroenterol Suppl. 1986;124:39-45.
23.- Ibironke GF, Olaleye SB, Balogun O, Aremu A. Effects of diets containing seeds of Garcinia Kola on gastric acidity in experimental ulceration in rats. Phytother Res. 1997; 11:312-313.
24.- Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38:421-464.
25.- Sokar Z, Gadhi CA, Benharref A, Jana M. Toxic effect of Herniaria cinerea DC. on the stomach, intestine, lung, and kidney of rats. J Ethnopharmacol. 2003;88:149-153.
26.- Dehghani F, Khozani TT, Panjehshahin MR, Karbalaedoost S. Effect of Teucrium polium on histology and histochemistry in rat stomach. Indian J Gastroenterol. 2005;24:126-127
27.- Kapicloglu S, Baki AH, Tekelioglu Y, Araz K. The effect of cola consumption on oral mucosa in rats. Dis Esophagus. 2000;13:69-71.
28.- Magaji RA, Ali MA, Mabrouk MA,Ibrahim NDG. The role of Clove on mucosal damage in albino aats: Book of Abstracts of the 1st International Neuroscience Conference. J Environmental Neurosci Biomed 2007;1:1.
Correspondence:
Ojo, Gideon B.
Department of Anatomy,
College of Health Sciences
Bowen University, P.M. B. 284,
Iwo. Osun State,
Nigeria.
Email: ojogbfavour @ yahoo.com