Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HISTOLOGIC AND ENZYMATIC COMPARISON BETWEEN PANGOLIN AND RAT LEFT MYOCARDIUM
Leke J. Medubi1, Ojuolape T. Aderinto2,
Ezekiel A. Caxton-Martins1
1Department of Anatomy, Faculty of Basic Medical Sciences,
College of Health Sciences, University of Ilorin
2Department of Physiology, Faculty of Basic Medical Sciences,
College of Medicine, University of Lagos.
Nigeria
Email: winsonleke @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2010;3:42-46
Comment of the reviewer Prof. Pilar Muñiz Rodríguez PhD. Department of Biochemist and Molecular Biology. Faculty of Sciences. Universidad de Burgos. Spain.
Comment of the reviewer Prof. Alberto Enrique D'Ottavio PhD. Professor and Researcher, Faculty of Medical Sciences, Rosario National University, Rosario. Argentina.
ABSTRACT
Pangolin is presumably a primitive mammal compared to rat. However, its cardiac contractile function is maintained longer than that of rat following cutting it away from the body immediately after euthanize. This investigation aim therefore to elucidate the microanatomy of the left ventricle in the pangolins in comparison with that of Wistar rats.
Biochemical enzyme quantification was also carried out in both mammals to evaluate differences in the levels of activities of the enzyme lactate dehydrogenase.
Following euthanasia and dissection along the thoracic wall, the left ventricles were recovered and divided into two parts. One part was fixed in 10% formal-saline and processed for paraffin embedding while the other was homogenized in sucrose and used for lactate dehydrogenase quantification.
Differences in the microanatomy of the left ventricles between pangolins and rats are reported essentially related with cardiomyocyte thickness, elastic fibers distribution, nuclear shapes, and perinuclear spaces. In addition, LDH activity appeared significantly higher in pangolins. Some of the detected differences could be correlated with animal size and perhaps, modes of life.
These preliminary results generate expectations about the future possibility of being pangolins suitable models for cardiovascular research. Further investigations are needed in this regard.
KEYWORDS: Cariomyocytes thickness nuclear shapes. Left ventricle. Lactate dehydrogenase.
RESUMEN:
El Pangolín es un mamífero, presumiblemente primitivo en comparación con las ratas. Sin embargo, su función contráctil cardiaca se mantiene más tiempo que en la rata después de la eutanasia por decapitación. Esta investigación tiene por objeto esclarecer la microanatomía del ventrículo izquierdo en los pangolines y compararla con la de ratas Wistar. También se llevó a cabo en los dos mamíferos una cuantificación bioquímica para evaluar las diferencias en los niveles de actividad del enzima lactato deshidrogenasa.
Tras la eutanasia se extrajo el ventrículo izquierdo medante disección a través de la pared torácica y fue dividido en dos partes. Una parte fue fijada en formal al 10% en solución salina y se procesó hasta la inclusión en parafina, mientras que el otro se homogenizó en sacarosa y fue utilizado para la cuantificación de lactato deshidrogenasa.
Las diferencias en la microanatomía de los ventrículos izquierdo entre las ratas y los pangolines son descritas y están esencialmente relacionadas con el grosor de los cardiomiocitos, la distribución de las fibras elásticas, las formas nucleares, y los espacios perinucleares. Además, la actividad LDH fue significativamente mayor en los pangolines. Algunas de las diferencias detectadas pueden ser correlacionados con el tamaño de los animales y tal vez, los modos de vida.
Estos resultados preliminares generan expectativas acerca de la posibilidad futura de que los pangolines puedan ser modelos adecuados para la investigación cardiovascular. Sin embargo, se necesitan más investigaciones al respecto.
PALABRAS CLAVE: Cardiomiocitos. ventrículo izquierdo. Lactato deshidrogenasa.
INTRODUCTION
It is well documented that the contractile function of the heart is an inherent property of the cardiomyocytes1-2. Using different animal models many workers have reported the micro-architecture of the cardiac muscle and blood vessels, which have helped to expand our knowledge of cellular and subcellular changes associated with cardiovascular function and dysfunction3-6. Cardiac extracellular matrix composed mainly of collagen and elastic fibers are among intrinsic factors that play critical roles in the contractile function of the heart. Well et al.,7 reported changes in mechanical properties and collagen cross-linking of the ovine thoracic aorta during perinatal development and postnatal maturation with its functional implication.
The rate of energy utilization is indicated by the level of activities of some enzyme of carbohydrate. In the myocardium, the process of glycolysis is the primary entry point for glucose for energy-yielding metabolism and lactate dehydrogenase (LDH) plays a relevant role8.
Taking into account: (a) that the choice of an animal for experimental study is, among other factors, influenced by exhibition of certain or unique morphofunctional characteristics peculiar to such an animal and (b) that this factor is, by itself, extremely useful for arousing curiosity and enabling the researcher to be more focused, pangolin was consequently signaled for the present cardiovascular investigation.
While pangolin is presumably a primitive mammal compared to rat, its cardiac contractile function appears more efficient than that of rat. This has been deduced from our direct observation of pangolin heart maintaining some degree of contractility longer than that of rat, when cut away from the body immediately after euthanasia.
Considering that no particular references have been found in specialized literature on this subject, we focused our investigation on the microanatomy of tree pangolin (Manis tricuspis) left ventricle and to carry out a parallel investigation in the rat for an appropriate comparison. In this regard, it is meaningful to outline that tree pangolin is native to equatorial Africa; from Senegal through Western Nigeria to Western Kenya and Southern Africa to Zambia9-10.
MATERIALS AND METHODS
Eleven animals, pangolin (n=6), rat (n=5) were used for this study. Pangolins of both sexes weighing between 1500- 3000g were sourced from local hunters in Asejire, Osun State and Iluke, Kogi State, Nigeria. The animals were brought to the Animal Holdings of Department of Anatomy, University of Ilorin, Nigeria. Pangolins were euthanized the same day they were brought into the laboratory since no modality has been developed to ensure its adaptation to captivity and moreover they are very violent in the night.
Wistar rats of both sexes weighing between 190g-220g were obtained from the Department of Biochemistry, University of Ilorin, Nigeria and brought to the Animal Holdings of the Department of Anatomy, University of Ilorin. They were fed with rat pellets (from Bendel Feed Limited, Ilorin, Nigeria) and provided water ad libitum
All animals were handled and maintained in accordance with the rules and guidelines of the animal right committee of the University Ilorin, Nigeria. The animals were thoroughly accessed, screened, and confirmed to be presumably free of any pathological conditions.
Animals were euthanized through cervical dislocation, the left thoracic wall immediately incised and the left ventricle identified, recovered, and divided into two parts. One of them was fixed in 10% formaldehyde-saline and processed for histological study. Sections of 7 µm were stained in hematoxylin and eosin, orcein and van Geison's stain, mounted and visualized in an Olympus binocular microscope interfaced with a JVC digital camera (S/No 10139477).
The micrographs were displayed on the screen of a desktop computer connected to the camera, captured on the screen, edited (with Microsoft picture manager), and appropriately labeled. The other part was homogenized in sucrose solution for biochemical measurement of serum LDH activity through a kinetic colorimetric assay11.
RESULTS
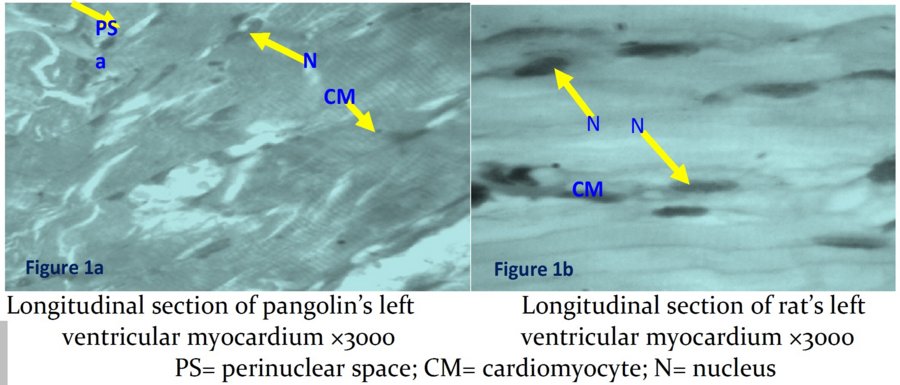
The micrographs showed cardiomyocytes thicker and more robust in pangolins than in rats. Nuclei were more elongated and slender in rats. Striations are faintly visible in pangolin section (Figures 1a and 1b).
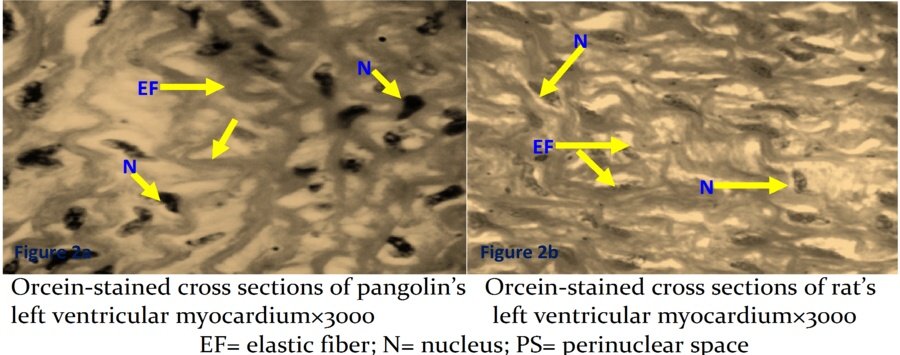
Both animals were rich in elastic fibers, as revealed by orcein stain. However, those of pangolins seemed to sweep out in thicker and more concentric fashion when viewed transversely (Figures 2a and 2b).
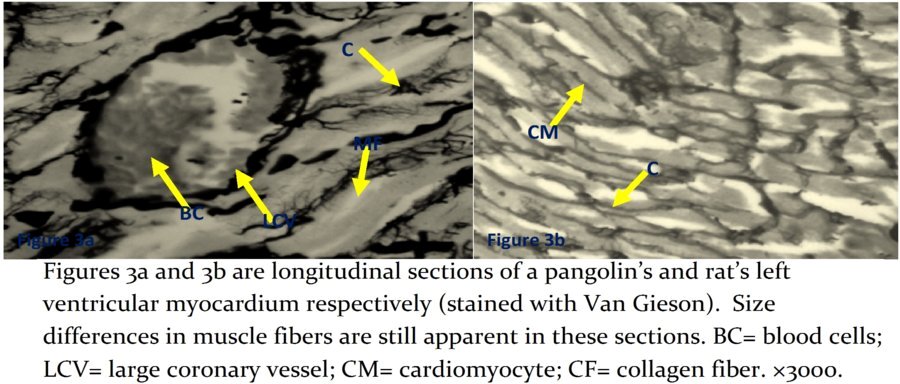
Collagen fibers appeared to be more widely distributed in rats than pangolins (Figures 3a and 3b).
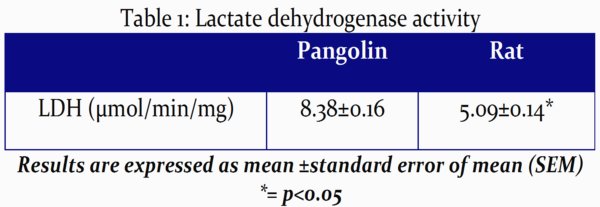
In accordance with Table 1, significant higher levels of LDH were registered in pangolins.
DISCUSSION
The rhythmical and apparently untiring contractile function of the heart can, in part, be understood by elucidating its structural and ultrastructural properties. Focusing our attention on the structural-functional correlation, light microscopy comparative analysis has been made between the micromorphology of the left ventricular myocardium of the tree pangolin and Wistar rat.
From this investigation, it is inferred that the microanatomical differences between the ventricular myocardium of the two mammalian species would mainly lie in the thickness of cardiomyocytes, and nuclear shapes. Sommer and Johnson12 had earlier shown variations in the density of the packaging of cardiomyocytes among different species. Indeed, the density of packaging had been shown to correlate with the mammalian heart size, that is to say, a relatively small mammal tends to have a comparatively loose packaging compared with a relative bigger mammal12.
The seemingly thicker cardiomyoctes in pangolins and the correspondingly thinner in rats suggest a positive correlation between animal size and myocardial fibers. The supposed functional adaptation for this is that, a larger heart requires a higher conduction velocity as well as greater force of contraction which is met in relatively thicker myocytes. Stated differently, the strength of a muscle fiber depends on its size. Among the factors that determine functional characteristic of cardiomyoctes are myoplasmic resistivity, membrane resistance, geometry of interconnection between tubular system and cell diameter12. Being all the other factors equal, the cardiomyocte diameter tends to define velocity of conduction and strength of contraction.
In this study, the relatively wider perinuclear spaces observed in pangolins suggest its degree of energy demand compared with rat. Perinuclear spaces are sites of mitochondria location13. Pangolins seem to live in the borderline of two extremes; so, it is totally passive during the day but exceptionally agile and active in the night. These two states require that its heart operates between the two states of metabolic demands. Its heart, which relies on fatty acid as a major source of energy, must markedly accelerate its beats during arousal. Within the long period of foraging in the night, extensive changes in heart rate, body temperature, and energy consuming processes can be expected. Such an altered requirement might be expected to result in changes in metabolic activities associated with mitochondria. Plasma free fatty acid concentration increases rapidly during arousal1, 14. This might account for the need of more mitochondria by pangolin's myocardium.
There are two different muscle components of LDH; muscle-LDH (M-LDH) and Heart-LDH (H-LDH)15 and it has been shown that M-LDH is usually present in tissues that are more active in anaerobic metabolism, such as white muscle, while H-LDH is found largely in tissues utilizing aerobic metabolism, such as cardiac muscle, which utilizes, fatty acid as a source of energy8.
The significant increase of LDH activity (P<0.05) in pangolin's left ventricular myocardium, here reported, lends some credence to some of the observable microanatomical differences in the left ventricular myocardium among pangolins and rats. Exceeding that H-LDH (isoform of LDH) found in the heart is essentially aerobically supportive, it is largely found in tissues utilizing aerobic metabolism such as the cardiac muscle which metabolizes fatty acid as a source of energy. In this sense, the inferred abundance of mitochondria and higher level of LDH activities could be positively correlated. This is to be expected in animals that often engage in rigorous activities where the heart muscle is especially capable of converting lactic acid to pyruvic acid and then using it for energy16. Further investigations are still needed in pangolins to determine its potentiality for being a suitable model for cardiovascular research.
REFERENCES
-
1.- Fonda ML, Herbener GH, Cuddihee RW. Biochemical and morphometric studies of heart, liver and skeletal muscle from the hibernating, arousing and aroused big brown bat, Eptesicus fuscus. Comp Biochem Physiol B. 1983; 76:355 -363
2.- Brady AJ. Mechanical property of isolated cardiac myocytes. Physiol Rev. 1991; 71: 413-428.
3.- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986; 58: 755-768
4.- Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol 1998; 506: 431-444
5.- Solaro RJ, Rarick HM. Troponin and tropomyosin: Proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res. 1998; 83: 471-480
6.- Fitzsimons DP, Patel JR, Moss RL Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filaments. J Physiol. 2001; 530: 263-272
7.- Wells SM, Langille BL, Lee JM, Adamson SL. Determinants of mechanical properties in the developing ovine thoracic aorta. Am J Physiol Heart Circ Physiol.1999; 277: H1385 - H1391
8.- Johnston DL Lewandoski ED. Fatty acid metabolism and contractile function in the reperfused myocardium. Multinucear NMR studies of isolated rabbit heart. Cir Res. 1991; 68:714-723
9.- Ciszek D. Manis gigantean (giant pangolin). Animal Diversity Web. University of Michigan Museum of Zoology. Available in Internet: http://animaldiversity.ummz.umich.edu/site/accounts/information/Manisgigantea.html [Accessed April 21 2008]
10.- Sodeinde OA, Adedipe SR. Pangolins in South -West Nigeria: Current Status and Prognosis. Oxyn. 1994; 28: 43-50.
11.- Babson AL, Babson SR. Kinetic Colorimetric Measurement of Serum Lactate Dehydrogenase Activity. Clinical Chemistry. 1973; 19: 766-769.
12.- Sommer JR, Johnson EA. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968; 36: 498-508.
13.- Challice CE, Viragh A. Ultrastructure of the mammalian heart. Ultrastructure in Biological System. Vol 6 New York (USA) Academic Press. 1973.
14.- Lewandoski ED. Nuclear magnetic resonance evaluation of metabolic and respiratory support of workload in intact rabbit heart. Cir Res 1992;70:576-782.
15.- Baba N, Shama H Histochemistry of lactate dehydrogenase in heart and pectorialis muscle of rat. J Cell Biol. 1971;51: 621-625.
16.- Guyton AC, Hall JE. Textbook of Medical Physiology, 10th Edition. 2003: 971-973.
CORRESPONDENCE:
Dr. Leke Jacob Medubi
Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences
University of Ilorin
PMB 1515, Ilorin
Nigeria
Email: winsonleke @ yahoo.com