Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
- Group A: A1 and A2;
- Group B: B1 and B2;
- Group C: C1 and C2.
PRENATAL EXPOSURE OF PREGNANT RATS TO CIGARETTE SMOKE AND NICOTINE: EFFECTS ON NITRIC OXIDE AND FASTING GLYCEMIA IN TREATED AND UNTREATED NEONATES WITH VITAMIN C
Obembe O.O1, Ukwenya V.O.2, Ige A.O3, Oyeyipo I.P 1 and Fasanmade A.A3
1Department of Physiology, College of Medicine. Osun State University, Osogbo, Osun,
2Departments of Anatomy, College of Health Sciences. Bowen University. Iwo, Osun State.
3Department of Physiology, College of Medicine. University of Ibadan
Nigeria
victorwyn @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2010;3:16-20
Comment of the reviewer reviewer Lourdes Lázaro Asegurado PhD. Department of Neumology. Complejo Asistencial Universitario de Burgos. Spain.
Comment of the reviewer Prof. Alberto Enrique D'Ottavio PhD. Professor and Researcher, Faculty of Medical Sciences, Rosario National University, Rosario (Argentina).
ABSTRACT
This paper studies the effects on plasma nitric oxide and fasting glycemia in neonates from pregnant rats exposed to cigarette smoke and nicotine, postnatally treated and untreated with vitamin C.
Fifteen female Wistar rats were divided into 3 groups of five rats each. Group A was exposed to cigarette smoke in an ad hoc chamber. Group B received 0.25 mg/kg body weight (BW) of nicotine while Group C served as control. After mating and gestation, litters from each group were randomly subdivided into two groups of 5 neonates each. Group A: A1 and A2, Group B: B1 and B2, Group C: C1 and C2.
Groups A1, B1 and C1 received 50 mg/kg BW Vitamin C for 4 weeks after birth while Groups A2 and B2 and C2 did not. Plasma nitric oxide and fasting glycemia were estimated in blood samples obtained from these animals.
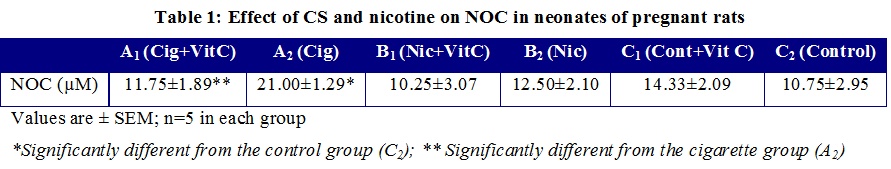
Results showed that the neonates from animals exposed to cigarette smoke had a significantly higher nitric oxide concentration (21.00±1.29 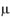 M) than those from not exposed ones (10.75±2.95
M) than those from not exposed ones (10.75±2.95 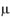 M) and a significantly lower fasting glycemia (61.00 +2.03 mg/dl) when compared with controls (75.50 + 3.73 mg/dl). In contrast, prenatal exposure to nicotine neither had a significant effect on nitric oxide concentration (12.50+2.10
M) and a significantly lower fasting glycemia (61.00 +2.03 mg/dl) when compared with controls (75.50 + 3.73 mg/dl). In contrast, prenatal exposure to nicotine neither had a significant effect on nitric oxide concentration (12.50+2.10 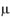 M) nor on fasting glycemia (74.17+3.48 mg/dl) when compared with controls (10.75+2.95
M) nor on fasting glycemia (74.17+3.48 mg/dl) when compared with controls (10.75+2.95 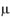 M) and (75.50+3.73 mg/dl), respectively.
M) and (75.50+3.73 mg/dl), respectively.
While the effects of cigarette smoke shown here could not be attributed to the pharmacological activity of nicotine, they may be related to the formation of smoking induced oxidative free radicals, since the administration of an antioxidant as vitamin C reversed them.
KEYWORDS: Nitric Oxide, Glycemia, Cigarette smoke, Nicotine, Vitamin C, Prenatal exposure, Rat.
RESUMEN: EXPOSICIÓN PRENATAL DE RATAS PREÑADAS A HUMO DE CIGARRILLO Y NICOTINA: EFECTOS SOBRE EL ÓXIDO NÍTRICO Y LA GLUCEMIA EN AYUNAS EN RECIÉN NACIDOS TRATADOS Y NO TRATADOS CON VITAMINA C
Este trabajo estudia los efectos en el plasma de óxido nítrico y la glucemia en ayunas en los recién nacidos de ratas embarazadas expuestas al humo del cigarrillo y la nicotina, tras el nacimiento tratados y no tratados con vitamina C.
Quince ratas Wistar fueron divididas en tres grupos de cinco ratas cada uno. El grupo A fue expuesto al humo del cigarrillo en una sala ad hoc. El grupo B recibió 0,25 mg / kg de peso corporal (PC) de la nicotina, mientras que el Grupo C sirvió como control. Después del apareamiento y la gestación, las camadas de cada grupo fueron subdivididos al azar en dos grupos de cinco recién nacidos cada uno. Grupo A: A1 y A2, el Grupo B: B1 y B2, Grupo C: C1 y C2.
Los Grupos A1, B1 y C1 recibieron 50 mg/kg de peso corporal de vitamina C durante 4 semanas después del nacimiento, mientras que los grupos A2 y B2 y C2 no lo recibieron. El óxido nítrico en plasma y la glucemia en ayunas se estimó en muestras de sangre obtenida de estos animales.
Los resultados mostraron que los recién nacidos de los animales expuestos al humo del cigarrillo tuvieron una concentración significativamente más alta de óxido nítrico (21.00±1.29 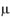 M) que las de los no expuestos (10.75±2.95
M) que las de los no expuestos (10.75±2.95 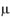 M) y una glucemia en ayunas significativamente menor (61.00 +2.03 mg/dl) en comparación con los controles (75.50 + 3.73 mg/dl). En contraste, la exposición prenatal a la nicotina no tuvo un efecto significativo sobre la concentración de óxido nítrico (12.50+2.10
M) y una glucemia en ayunas significativamente menor (61.00 +2.03 mg/dl) en comparación con los controles (75.50 + 3.73 mg/dl). En contraste, la exposición prenatal a la nicotina no tuvo un efecto significativo sobre la concentración de óxido nítrico (12.50+2.10 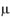 M), ni sobre la glucemia en ayunas (74.17+3.48 mg/dl) en comparación con los controles (10.75+2.95
M), ni sobre la glucemia en ayunas (74.17+3.48 mg/dl) en comparación con los controles (10.75+2.95 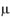 M) y (75.50+3.73 mg/dl), respectivamente.
M) y (75.50+3.73 mg/dl), respectivamente.
Si bien los efectos del humo del cigarrillo se muestran aquí no se puede atribuir a la actividad farmacológica de la nicotina, que puede estar relacionado con la formación del hábito de fumar oxidativo inducido por radicales libres, ya que la administración de un antioxidante como la vitamina C que invierte.
PALABRAS CLAVE: Óxido nítrico, glucemia, el humo del cigarrillo, la nicotina, la vitamina C, la exposición prenatal, Rata.
INTRODUCTION
Cigarette smoking (CS) continues to be a widespread public health problem; of the nearly 1 billion smokers worldwide, half are likely to die of smoking-related diseases. It is still unclear whether the injurious effects of cigarette smoking in pregnancy are due to nicotine, and if so, what the effects are.
The effects of CS on the NO pathways and Nitric Oxide Synthase (NOS) isoenzymes are controversial and may vary according to the disease, model or location of the NOS1. For example, while exhaled NO has been shown to be decreased in humans after acute cigarette exposure, inducible nitric oxide synthase (iNOS) mRNA expression increased in the lungs of rats exposed to cigarette smoke, while neuronal nitric oxide synthase (nNOS) showed a longer term increase in both transcription and translation1. CS has been shown, however, to cause a reduction in nitrite concentration and iNOS expression in a murine lung epithelial cell line in vitro2.
In contrast, Comhair et al3 showed no change in iNOS expression in airway cells from healthy subjects exposed to CS. The effects of CS on NOS in the vasculature has shown a reduction in endothelial constitutive NO (ecNOS) in the pulmonary vessels in vitro and in vivo, genetic variation in man, while vascular intimal thickening and up-regulated iNOS has been described in mice3. These seemingly contradictory effects are probably explained in part by the different tissue situations and also by variation in the constituents of the CS3.
While neonatal mortality has greatly declined through improved pediatric care, unfortunately there seems to be no similar reduction in the incidence of infant morbidity and in the occurrence of childhood developmental disorders of the brain and intellect. The reasons for this are manifold; however, several investigations have now been able to provide ample evidence that pre-term and low birth weight infant carries the major risk of suffering from developmental delay, whether mental or physical. In addition, more serious conditions such as mental retardation, retinopathy of prematurity, broncho-pulmonary dysplasia, brain hemorrhage, spasticity, cerebral palsy, blindness, deafness, autism and epilepsy are a frequent consequence of pre-term birth, which has been linked to cigarette smoking during pregnancy4
The health effects of sub-chronic exposure to low levels of wood smoke in rats were examined by Yohannes et al 5. They observed that pulmonary functions, especially CO2 diffusing capacity and pulmonary resistance were somewhat affected in high exposure group. Also mild chronic inflammation and squamous metaplasia were observed in the larynx of exposed groups. The severity of these increased with smoke concentration and length of exposure. Also Xiu et al6 studied the effects of cigarette smoke on degranulation and nitric oxide (NO) production by mast cells and epithelial cells, and found that exhaled NO is decreased in active and passive smokers, suggesting that it inhibits NO production. Since NO is the most important vasodilator synthesized by blood vessels, this study was designed to examine whether prenatal exposure to cigarette smoke affects plasma NO with alteration of blood glucose level; and to ascertain the role of nicotine.
MATERIALS AND METHODS
Animals
Fifteen (15) healthy female rats, weighing 250-300g, were obtained from the central Animal House, College of Medicine and housed in well ventilated cages (5 animals per cage) in the Animal House of the Department of Physiology, University of Ibadan. They were exposed to 12 hour light and 12 hour-dark cycle, relative humidity 50-53% and a temperature range of 26-28°C. The animals were fed rat pellets and tap water ad libitum and were allowed two (2) weeks of acclimatization.
Ovulation/Mating: Ovulation was induced by administration of diethylstilbestrol (stilboestrol ®) - an orally active synthetic estrogen-. At 09:00 am, 0.042 mg/ kg BW of diethylstilbestrol was administered using an oral cannula. Ovulation was confirmed by vaginal smear as described by Marcondes et al 7.
Mating was naturally allowed by introducing a male rat into each group. Estrus cycle occurred every 3-4 days in rats and gestation lasted 21-23 days, with notable pregnancy at about 14 days. Each female rat had between 6 and 15 litters.
Cigarette Smoke Exposure:
London king size (menthol) cigarettes (London Tobacco Company) were used. Each cigarette contained 14.9 mg tar and 1.2 mg nicotine. Rats were exposed to smoke from an idling cigarette in an ad hoc chamber over a period of 30 minutes per day (09:00 am-09:30 pm), from day 0 to day 20 of gestation. Four (4) cigarettes were used for each animal group exposure per day. Food and water were removed from chambers before start of exposure, and then replaced after exposure.
Nicotine administration
0.25mg/kg BW of standard nicotine was daily administered by intramuscular injection from day 0 to day 20 of gestation.
Vitamin C administration
50 mg/kg BW of vitamin C (Tuyil Pharmaceutical Company, Ilorin, Kwara State) was daily and orally administered through an oral cannula during six (6) weeks from 4 weeks post-birth.
Estimation of Nitric Oxide (NO)
Blood samples were collected by left cardiac puncture after ether anesthesia, allowed to clot for 15 minutes and centrifuged at 2000 sign g for 10 minutes at room temperature. Serum specimens were portioned into polypropylene tubes. All biological specimens were stored at -10°C until analyzed.
Plasma Nitric Oxide was estimated by the Griess Reagent System, which measures nitrite (NO2), one of two primary, stable and nonvolatile breakdown products of NO. This assay relies on a diazotization reaction originally described by Griess in 18798. Absorbance was read at 540 nm in a spectrophotometer (Pharmacia Biotech, Uppsala, Sweden). Average absorbance value of each experimental sample was calculated and its concentration determined by comparison with the Nitrite Standard reference curve.
Determination of Fasting Glycemia (G0)
G0 was estimated ten (10) weeks post-birth. Blood samples were collected from the tail-tips of conscious rats after 12 hours overnight fast. Glucometer (Acu-chek® Johnson-Johnson, California, USA) and compatible glucometer strips were used for these determinations.
Experimental Design
Fifteen (15) female Wistar rats were divided into 3 groups of 5 rats each. After mating and confirmation of pregnancy, Group A were exposed to cigarette smoke (CS), Group B received nicotine while Group C served as control. After parturition, neonates were subdivided as follows:
Groups A1, B1 and C1 further received 50 mg/kg BW of vitamin C while Groups A2, B2 and C2 did not. Animals were checked once in the morning and once in the late afternoon at least 6 hour apart 7 days a week for detecting any clinical signs of abnormality, morbidity, or mortality as outlined by Jaci et al 9.
All procedures in this study conformed to the guiding principles for research involving animals as recommended by the Declaration of Helsinki and the Guiding Principles in the Care and Use of Animals10 and were approved by the Departmental Committee on the Use and Care of Animals.
Statistical Analysis
Data are expressed as mean ± SEM and analyzed using the Student's t-test and ANOVA when necessary. P< 0.05 was accepted as statistically significant.
RESULTS
Effect of CS and nicotine on NO Concentration (NOC)
Neonates of pregnant rats exposed to CS (A2) had a statistically significantly higher NO concentration than controls and A1. No significant differences were registered with regards to prenatal exposure to nicotine (Table 1)
Effect of CS and nicotine on G0
Results showed that neonates of rats exposed to CS had significantly lower G0 when compared with controls and A1 (Table 2). G0 in nicotine administered animals was not significantly different from controls.

DISCUSSION
Results showed that neonates exposed to CS in the intrauterine life had a significantly higher NO concentration than those exposed to nicotine. This corroborates the report of Yang et al11 which claimed that passive smoking decreases NOS activity and increased the NO content of mice. As the prenatal exposure to nicotine administration showed no significant difference in NO concentration in relation with the control group, the effect CS could not be attributed to the pharmacological activity of nicotine. Numerous other toxins in cigarette smoke adversely affect the placental circulation and/or fetal physiology and development12.
However, it may be related to the formation of smoking induced oxidative free radicals since the administration of an anti-oxidant as vitamin C reversed that effect. This agrees with reported by Seller and Bnait13 about the detrimental effects of cigarette smoke in both high and low tar cigarettes.
Previous research has demonstrated the ability of vitamin C administration, taken by nonsmokers two hours prior to being exposed to CS, to reduce the free radical damage, LDL oxidation associated with exposure to CS and the smoke-induced decline in total antioxidant defense. These beneficial effects were not observed in nonsmokers under normal conditions14. Researchers have also shown that vitamin C may enhance endothelial function by promoting the synthesis of NO or by preventing its inactivation by scavenging superoxide radicals14. This outcome is consistent with ours, as the NO concentration of the control group is found to increase following vitamin C administration.
The NO concentration of the group exposed to CS and there after administered vitamin C was found to be significantly lower than those not administered vitamin C. There was no significant difference between the NO level of the nicotine and nicotine + vitamin C groups suggesting that prenatal exposure to intramuscular nicotine injection (0.25mg kg/b.w) had no effect on the formation of oxidative free radicals endogenous NO. Also, the NO concentration difference between cigarette + vitamin C group and the control group was not significant, indicating that vitamin C administration reversed this damaging effect of CS. This agrees with the work of Yang et al 11 who reported that vitamin E (an antioxidant) improved the learning and memory ability of offsprings whose mothers were exposed to tobacco smoke during pregnancy.
Further studies are needed to see if the effect of CS during intra uterine life on NO level will be blocked by anti- oxidants.
However, this work also showed that prenatal exposure to CS caused a significant reduction in G0 when compared with the control group. Glucose is stored in muscle tissue as glycogen, thereby contributing to body weight. Lower G0 in neonates of prenatally exposed rats could be a factor in low-birth weight observed in women who smoke during pregnancy15 and might be due in part to the finding that maternal smoking reduces endothelium-dependent nitric oxide-mediated relaxation in uterine small arteries16.Recent findings by Malene et al also indicate that maternal smoking reduces eNOS activity in the fetal vascular bed, contributing to retarded fetal growth caused by the reduction of vasodilatory capacity17.
On the other hand, the difference between G0 of those exposed to nicotine administration and the control group was not significant. Thus, the effect of prenatal exposure to CS on BGL could again not be attributed to the pharmacological activity of nicotine, but may be related to the formation of smoking induced oxidative free radicals.
Administration of vitamin C has shown to cause a significant increase in G0 of the control group. This increase is consistent and statistically significant in all groups. Vitamin supplements thus increases G0. This appears congruent with Donovan et al18 who revealed that the administration of vitamin C to overnight fasted dogs resulted in significant elevation of G0 attributed to significant reduction in plasma insulin levels mainly due to impaired insulin release. A likely explanation for the impaired insulin release could be that competition between glucose and vitamin C for transport into the islet cells may have slowed glucose entry, thereby impairing the glucose-sensing apparatus of the islet cells18.
Summing up, this study reveals that prenatal exposure to passive smoke from cigarettes causes an increase in plasma NO and a decrease in G0 of offspring and that these effects may be not associated with the pharmacological activity of nicotine, since exposure to nicotine did not significantly alter these variables. Furthermore, postnatal administration of the anti-oxidant vitamin C to the offspring reversed the effects induced by prenatal exposure to CS.
REFERENCES
- 1. Yates DH. Breen H. Thomas PS. Passive smoke inhalation decreases exhaled nitric oxide in normal subjects. Am J Respir Crit Care Med. 2001; 164:1043-1046.
2. Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med.1995; 152:609-612
3. Comhair SA, Thomassen MJ, Erzurum SC. Differential induction of extracellular glutathione peroxidase and nitric oxide synthase 2 in airways of healthy individuals exposed to 100% O2 or cigarette smoke. Am J Respir Crit Care Med. 2000; 23:350-4.
4. Crawford MA, Doyle W, Leaf A, Leighfield M. Nutrition and neuro-developmental disorders. Nutrition and Health.1993; 9:81-97.
5. Tesfaigzi Y, Singh SP, Foster JE, Kubatko J, Barr EB, Fine PM, McDonald JD, Hahn FF, Mauderly JL. Health effects of subchronic exposure to low levels of wood smoke in rats. Toxicol Sci. 2002;65:115-125.
6. Wei XM, Kim HS, Kumar RK, Heywood GJ, Hunt JE, McNeil HP, Thomas PS.
Effects of cigarette smoke on degranulation and NO production by mast cells and epithelial cells. Respir Res. 2005;6:108.
7. Marcondes FK, Bianchi FJ Tanno AP. Determination of the Estrous Cycle Phase of Rats: Some Helpful Considerations. Braz J Biol. 2002;62(4a):609-614.
8. Muijsers RB, van Den Worm E, Folkerts G, Beukelman CJ, Koster AS, Postma DS, Nijkamp FP. Apocynin inhibits peroxynitrite formation by murine macrophages. Br J Pharmacol. 2000;130:932-936.
9. Vanheest JL, Rodgers CD. Effects of exercise in diabetic rats before and during gestation on maternal and neonatal outcomes. Am J Physiol Endocrinology Metab.1997; Vol. 273, Issue 4, E727-E733.
10. World Medical Association, American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002; 283:R281-R283.
11. Yang J, Jiang LN,Yaun ZL, Zheng YF, Wang L, Ji M, Shen ZQ, Wang XM, Ma Q, Xi ZG, Li JW. Impacts of passive smoking on learning and memory ability of mouse offsprings and intervention by anti-oxidants. Biomed Environ Sci.2008;21(2):144-149
12. Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry. 2003;160:323-333.
13. Mary J. Seller MJ, Bnait KS. Effects of tobacco smoke inhalation on the developing mouse embryo and fetus. Reproductive Toxicology.1995; 9:449-459.
14. Valkonen MM, Kuusi T. Vitamin C prevents the acute atherogenic effects of passive smoking. Free Radic Biol Med. 2000; 28:428-36.
15. Chelchowska M, Lewandowski L, Ambroszkiewicz J, Swiatek E, Gajewska J, Oltarzewski M, Laskowska-Klita T. The effect of tobacco smoking during pregnancy on concentration of pro-hepcidin and some parameters of iron metabolism in matched-maternal cord pairs. Przegl Lek. 2008; 65:474-478.
16. Andersen MR, Uldbjerg N, Stender S, Sandager P, Aalkjær C. Maternal smoking and impaired endothelium-dependent nitric oxide-mediated relaxation of uterine small arteries in vitro. Am J Obstet Gynecol. 2010 Oct 23. [Epub ahead of print].
17. Andersen MR, Simonsen U, Uldbjerg N, Aalkjaer C, Stender S. Smoking cessation early in pregnancy and birth weight, length, head circumference, and endothelial nitric oxide synthase activity in umbilical and chorionic vessels: an observational study of healthy singleton pregnancies. Circulation. 2009;119:857-864.
18. McGrowder D, Ragoobirsingh D, Dasgupta T. The enhancement of the hyperglycemic effect of S-nitrosoglutathione and S-nitroso-N-acetylpenicillamine by vitamin C in an animal model. BMC Pharmacol. 2002;2:18.
ACKNOWLEDGEMENT
We appreciate Dr O.G. Arinola of the Department of Chemical Pathology, University College Hospital, Ibadan, Nigeria for his whole hearted assistance and wealth of knowledge for providing the technical know-how for this work.
CORRESPONDENCE:
Ukwenya Victor Okoliko
Department of Anatomy, Faculty of Basic Medical Sciences,
College of Health Sciences,
Bowen University, Iwo
Osun State. Nigeria
Email: victorwyn @ yahoo.com
Comment of the reviewer Lourdes Lázaro Asegurado PhD. Department of Neumology. Complejo Asistencial Universitario de Burgos. Spain.
This study is very interesting because it sheds light on the pathophysiology of the effects of environmental smoke snuff in the fetus during pregnancy and could be extrapolated to snuff consumption. Vascular damage would be related to exposure to smoke oxidants snuff and not to nicotine. The same could be said about the negative effects on intrauterine growth by lowering fasting glucose.
This finding is of particular interest to support the nicotine replacement therapy during pregnancy and safe therapy to help quit smoking to pregnant women who do not get it with behavioral therapy.
Comment of the reviewer Prof. Alberto Enrique D'Ottavio PhD. Professor and Researcher, Faculty of Medical Sciences, Rosario National University,
Rosario (Argentina).
The article by Obembe OO et al is devoted to study the effects on nitric oxide and fasting glycemia in treated and untreated neonate rats with vitamin C whose mothers were prenatally exposed to cigarette smoke and nicotine. In general, this topic keeps scientific significance in accordance with the specialized bibliography. As its final version did not deserve essential and/or formal objections, the article may be accepted for publication.
Received July 10, 2010. Received reviewed December 4, 2010
Published: December 8, 2010
