Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
HISTOLOGICAL EFFECTS OF CHRONIC ADMINISTRATION OF AQUEOUS EXTRACT OF PHYLLANTHUS AMARUS ON THE STOMACH AND THE DUODENUM OF WISTAR RATS
J.O. Adjene1, I.E. Abudu 1, E.U. Nwose 2
1Department of Anatomy, School of Basic Medical Sciences,
University of Benin. Nigeria
2Institute of Clinical Pathology & Medical Research. South West Pathology.
Albury, NSW. Australia
ezekiel.nwose @ gsahs.health.nsw.gov.au
Rev Electron Biomed / Electron J Biomed 2011;1:9-13.
Comment of the reviewer Daniel Al Kassam PhD. Biochemistry. Complejo Asistencial Universitario de Burgos. España.
Comment of the reviewer Prof. Dr. Alberto Enrique D'Ottavio. Catedrático de Histología y Embriología. Facultad de Ciencias Médicas. Universidad Nacional de Rosario. Rosario. Argentina
ABSTRACT
Background: Phyllanthus amarus is a herbal agent commonly used for the treatment of several diseases including gastric ones. A systematic study about its toxic effects when chronically and excessively administered is still lacking.
Aim: This study investigates the histological effects of chronic administration of the herb on the stomach and the duodenum of adult Wistar rats of both sexes.
Material and methods: Rats, with average weight of 200g, were divided into three groups of eight animals each: A (P amarus at a single dose of 400mg/kg body weight daily for thirty days through an orogastric tube); B (P. amarus at a single dose of 800mg/kg body weight daily through the same route and period), and C (controls, receiving equal volume of distilled water in identical conditions to A and B). Rats were fed with grower's mash and received water ad libitum. Animals were euthanized by cervical dislocation on the thirty-one day of the experiment and the stomach and the duodenum were immediately dissected out and fixed in 10% formaldehyde- saline for routine histological techniques. Results: Rats in the treated groups showed some distortions in the epithelial cells of the stomach and the duodenum and signs of inflammation, when compared with controls.
Conclusion: This report provides evidence that ethnomedicinal practice involving chronic administration of Phyllanthus amarus at high doses has potential adverse histological effect in determined digestive organs. It is likely that the herb may be contraindicated in gastro-ulcer patients. This warrants further studies to establish or rule out any untoward side-effect on bowel response and/or digestive dysfunctions.
Keywords: Antioxidant toxicity, digestive tract, ethnomedicinal practice, histological effects, Phyllanthus amarus
RESUMEN: EFECTOS HISTOLOGICOS DE LA ADMINISTRACIÓN A LARGO PLAZO DEL EXTRACTO ACUOSO DE PHYLLANTHUS AMARUSSOBRE EL ESTÓMAGO Y EL DUODENO DE RATAS WISTAR
Antecedentes: Phyllanthus amarus es un agente a base de hierbas de uso común para el tratamiento de varias enfermedades incluyendo los trastornos gástricos. Aun está pendiente un estudio sistemático sobre sus efectos tóxicos cuando se administran en altas dosis por largo periodo de tiempo.
Objetivo: El objeto de este estudio es investigar los efectos de la administración crónica de esta hierba sobre la histología del estómago y duodeno de ratas Wistar adultas de ambos sexos.
Material y métodos: Las ratas, con un peso promedio de 200 g, se dividieron en tres grupos de ocho animales cada uno. El grupo A recibió P. amarus en dosis única de 400 mg/kg de peso corporal al día durante treinta días mediante sonda gástrico, el grupo B recibió P. amarus en una sola dosis de 800mg/kg de peso corporal al día con el mismo procedimiento y período, y el grupo C fue de ratas control, que recibió el mismo volumen de agua destilada en idénticas condiciones que A y B. Las ratas fueron alimentadas con pienso especial para ratas y agua ad libitum. Los animales fueron sacrificados por dislocación cervical en el día treinta y uno del experimento y el estómago y el duodeno fueron disecados e inmediatamente fijados en formol al 10% en solución salina para técnicas histológicas de rutina. Resultados:En los grupos tratados, las ratas mostraron algunas distorsiones en las células epiteliales del estómago y el duodeno y signos de inflamación, en comparación con los controles.
Conclusión: Este trabajo present evidencias de que la práctica etnomedicinal de administración a largo plazo de Phyllanthus amarus en altas dosis tiene potencial efecto adverso histológico en determinados órganos digestivos. Es probable que esta hierba puede estar contraindicada en pacientes con úlcera gastrointestinal. Se requieren estudios adicionales para establecer o descartar efecto secundarios en la respuesta intestinal y / o trastornos digestivos.
Palabras clave: Toxicidad antioxidante. Tracto digestivo. Etnomedicina práctica. Cambios histológicos, Phyllanthus amarus
INTRODUCTION
Herbal medicines are widely perceived by the public as being free from side effects. However, herbal therapies have not been effusively researched or standardized to enable clinical application1. Indeed, some of the herbal agents are unknown in the Western world and concerns regarding side effects in modern evidence-based medicine practice are major2. Hence, safety of herbal medicines is still an issue worldwide2-4.
Phyllanthus amarus (P. amarus) is an ethnobotanical plant that is distributed in almost all tropical countries and regions including America, India and Nigeria. It is a common weed, which grows well in moist, shady and sunny places. It is known by several other names such as carry-me-seed, chanca piedra, and quinine weed to mention a few5. P. amarus is believed to possess antioxidant, antiseptic, and stomachic properties among others, and it is used accordingly in many countries6-10. It is particularly used traditionally in the treatment of several bowel diseases including diarrhea, dysentery and ulcers5,10-11.
Plants contain hundreds of constituents and some of them may elicit toxic side effects. Phytochemically, P. amarus contains alkaloids, antioxidants (including flavonoids, phenols and polyphenols) and lignans5. All of these chemical components have their useful as well as toxic effects. The toxic effects of P. amarus has been suggested in literature12-13. However, histological perspectives are yet to be elaborated.
In mammals including humans and rats, the digestive tract is composed of tubular organs that are joined together sequentially from the mouth, esophagus, stomach, small intestine (including duodenum, ileum and jejunum) and large intestine to the anus. Along this continuum of the digestive tract, chemical digestion occurs minimally in the mouth, predominantly in stomach and duodenum, and is almost complete at arriving to the ileum. It is known that digestion of certain foods such as vegetables is often incomplete14. It is also known that while food is emptied from the mouth and oesophagus as quickly as it is swallowed, emptying of the stomach and small intestine in digestive process is relatively slow and not consciously controlled. Yet, it is varied for different types of food. For instance, proteins are emptied slower that carbohydrates15. The implication is that any toxic content of food may have very limited time to affect the mouth and oesophagus, the potential risk on the stomach or small intestine is impacted by the relative longer time the food stays in these two regions.
Therefore, the stomach and duodenum are logically the regions of the digestive tract that may be most affected by any toxic component of foods. Since P. amarus posses some potentially toxic phytochemicals5, it would be worthwhile to explore the effects of chronic administration of the herb on the microanatomy of the digestive tract by examining the stomach and duodenum.
MATERIALS AND METHODS
Animals and ethical concerns: This study is part of a project on the histological effects of P amarus research. Twenty-four adult Wistar rats of both sexes, with average weight of 200g, were equally and randomly assigned into three groups (n=8 each): two treatment groups [A] and [B] and one, untreated or control [C]. The School of Basic Medical Sciences, University of Benin granted approval before the work began.
The animal care and use ethics was in compliance to the Animal Holdings protocol overseen by the head of department through the Animal Holding unit. Rats were obtained and maintained in the Animal Holdings of the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria. The animals were fed with grower's mash obtained from Edo Feeds and Flour Mill Limited, Ewu, Edo State, Nigeria and received water ad libitum.
Preparation and administration of P. amarus: The P. amarus leaves were obtained in Benin City. The leaves were cleaned, oven-dried at 50°C and macerated into dry powder. The powder was extracted with distilled water using Soxhlet apparatus and concentrated by rotary evaporator at 65° C. It was then transferred into a suitable container and freeze dried ready for the experiment. All preparations were performed at the Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, Benin city, Edo State, Nigeria.
Treatment of animals: In the main research protocol, animals in group A received the aqueous extract of P amarus at a single dose of 400mg/kg body weight daily for thirty days through the orogastric tube whilst animals of group B received a single dose of 800mg/kg body weight daily via the same route and the same period. Animals in group C received equal volume of distilled water, for the same period and through the same route of administration. Rats were euthanized by cervical dislocation on the thirty-one day of the experiment and the stomach and the duodenum were immediately dissected out and fixed in 10% formaldehyde- saline for routine histological techniques.
Histological study: Specimens were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 7 µm thick were obtained using a rotatory microtome. The deparaffinized sections were stained routinely with haematoxyline and eosin (H&E). Photomicrographs were obtained using research photographic microscope in the Department of Anatomy, School of Basic Medical Sciences, University of Benin, Benin city, Edo State, Nigeria.
Selection criterion: It has been speculated that toxic effects could be independent of concentration16. In earlier report on kidneys, we registered no remarkable difference between the treated groups17. Sections were discretionally selected from groups [B] and [C] only for comparison.
RESULTS
The photomicrograph of the stomach section from the control group showed normal histological features with the mucosa lined with simple columnar epithelial cells, lamina propria with some highly packed glandular secretory cells. The muscularis mucosa of the stomach was well noticed in the control section (Fig.1a). The sections of the stomach treated with P amarus revealed some varying degree of distortion in the epithelial layer. There were obvious sign of proliferation, and atrophic changes in the treated stomach sections (Fig. 1b).
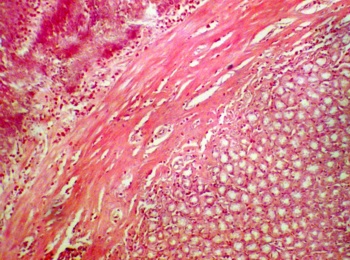 a. Control section of the stomach (H&E x10). |
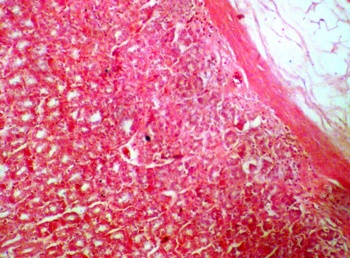 b.Section of stomach treated with P.amarus 800mg/kg (H&Ex10) |
The duodenum section from the control group [C] also evidenced normal histological features. There were indications of the presence of Brunner's gland and other cells lining the duodenum (Fig. 2a). The duodenum treated with P amarus showed signs of erosion and inflammation, which are suggestive of ulcerations. The photomicrograph revealed some distortion and disruption in the epithelial lining, which may be suggestive of atrophic duodenitis (Fig. 2b)
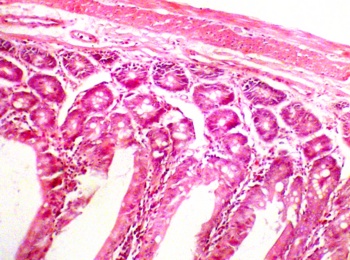 a. Control section of the duodenum (H&Ex10) |
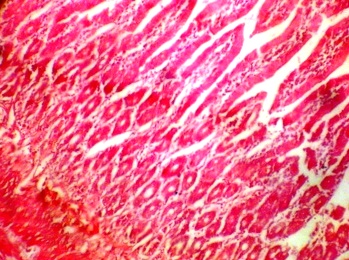 b. Section of duodenum treated with P.amarus 800mg/kg (H&Ex10) |
Limitations: The observations from this study have limitations. Gender differences among the rats within groups were not investigated. As explained in selection criteria, difference between groups A and B were not considered in this particular evaluation. Only the group B animals have been compared with control group C in this study.
DISCUSSION
Since administration of P amarus was the only differential treatment between treated and untreated groups, we guess that the registered toxic effects (Figures 1 and 2) may be attributed to the phytochemical content of P amarus.
These results not only were congruent with a former report related with the toxic effects of red pepper administration on the cytoarchitecture of the stomach18, and with others found in the available literature12-13. In contrast, our findings differ from those of Lawson-Evi et al,19 who reported no toxic effect or anatomical changes in digestive organs with P. amarus. However, it must be outlined that these authors employed low doses of the plant.
Considering the known health benefits of the herbal agent8-11, 20-22 it may be also guessed that the excessive and prolonged intake of P amarus resulted in the aforesaid toxic effects on the epithelial lining. One of the major components of P amarus is the antioxidant tannin, basically a phenolic compound, whose excessive and/or indiscriminate consumption can be toxic23. P. amarus also contains alkaloids and lots of other antioxidants5, 9, which has been administered in this study to apparently healthy animals. Thus, our observation is consistent with the notion that antioxidants may be associated with toxicities, particularly when arbitrarily consumed24.
Exceeding that antioxidants are essential for alleviation of oxidative stress, indiscriminate intake of alkaloids and antioxidant constituents of P. amarus may induce oxidative stress25-26. Our results are indicative that arbitrary chronic and/or excessive consumption of P. amarus may be harmful for animals.
In clinical practice, patients with peptic ulcer are advised to abstain from pepper. Taking into account the similar toxic effects produced on the cytoarchitecture of the stomach by red pepper administration18, and the results here reported, a rational basis is provided to hypothesize that ethnomedicinal practice involving the use of P. amarus may be contraindicated in peptic ulcer patients, especially if the herbal agent is required to be administered in high dosage. Summing up, it calls for caution and discretion as occurs with other medicines.
CONCLUSION
This study presents histological evidence supporting that chronic and excessive administration of P. amarus has potential adverse effect on the microanatomy of the stomach and the duodenum. The hypothetical implication is that the function of digestion and absorption may be adversely affected by P. amarus. Last but nor the least, we hypothesize the P amarus may be contraindicated in stomach ulcer patients. Further analyses are recommended to verify this hypothesis.
Acknowledgement
This work is part of Josiah Adjene's main research supported by the University of Benin. Some of the laboratory work for this particular report was done by Imuentinyan Abudu as part of his undergraduate study. Dr. Uba Nwose contributed the Translational Biomedical Science concepts. The authors gratefully acknowledge the support from various staff of the department of Anatomy of the University of Benin, Nigeria.
REFERENCES
-
1. Sarris J, Kavanagh DJ, Byrne G. Adjuvant use of nutritional and herbal medicines with antidepressants, mood stabilizers and benzodiazepines. J Psychiatr Res 2010;44:32-41.
2. Pieroni A, Sheikh QZ, Ali W, Torry B. Traditional medicines used by Pakistani migrants from Mirpur living in Bradford, Northern England. Complement Ther Med 2008; 16:81-6.
3. Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 2000;33:179-89.
4. Calapai G. European Legislation on Herbal Medicines: A Look into the Future. Drug Safety 2008;31:428-31.
5. Fernand VE. Initial characterization of crude extracts from Phyllanthus amarus schum. and thonn. and Quassia amara L. using normal phase thin layer chromatography: Louisiana State University; 1998.
6. Calixto JB, Santos AR, Cechinel-Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev 1978;18:225-58.
7. Faremi TY, Suru SM, Fafunso MA, Obioha UE. Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol 2008;46:2658-64.
8. Harikumar KB, Kuttan G, Kuttan R. Inhibition of Viral Carcinogenesis by Phyllanthus amarus. Integr Cancer Ther 2009;8:254-60.
9. Naaz F, Javed S, Abdin MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. on aflatoxin B1-induced liver damage in mice. J Ethnopharmacol 2007;113:503-9.
10. Odetola AA, Akojenu SM. Anti-diarrhoeal and gastro-intestinal potentials of the aqueous extract of Phyllanthus amarus (Euphorbiaceae). Afr J Med Med Sci 2000;29:119-22.
11. Harikumar KB, Kuttan R. An extract of Phyllanthus amarus protects mouse chromosomes and intestine from radiation induced damages. J Radiat Res (Tokyo) 2007;48:469-76.
12. Manjrekar AP, Jisha V, Bag PP, et al. Effect of Phyllanthus niruri Linn. treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol 2008;46:514-20.
13. Adedapo AA, Adegbayibi AY, Emikpe BO. Some clinico-pathological changes associated with the aqueous extract of the leaves of Phyllanthus amarus in rats. Phytother Res 2005;19:971-6.
14. Tovar J, Bjorck IM, Asp N-G. Incomplete digestion of legume starches in rats: a study of precooked flours containing retrograded and physically inaccessible starch fractions. J Nutr 1992;122:1500-7.
15. Your digestive system and how it works. 2008. (Accessed 13th August, 2010, at http://digestive.niddk.nih.gov/ddiseases/pubs/yrdd/.)
16. Campos AH, Schor N. Phyllanthus niruri inhibits calcium oxalate endocytosis by renal tubular cells: its role in urolithiasis. Nephron 1999;81:393-7.
17. Adjene JO, Nwose EU. Histological effects of chronic administration of Phyllanthus amarus on the kidney of adult Wistar rat. North Am J Med Sci 2010;2:193-5.
18. Kendabie KO, Adjene JO. Histological studies of the effects of red pepper on the stomach of adult wistar rats. Rev Electron Biomed / Electron J Biomed 2007;3:13-7. Available online at: http://biomed.uninet.edu/2007/n3/kendabie.pdf. Accessed 19th August, 2010
19. Lawson-Evi P, Eklu-Gadegbeku K, Agbonon A, et al. Toxicological assessment on extracts of Phyllanthus amarus Schum and Thonn. Sci Res Essays 2008;3:410-5.
20. Rao MV, Alice KM. Contraceptive effects of Phyllanthus amarus in female mice. Phytother Res 2001;15:265-7.
21. Adeneye AA, Amole OO, Adeneye AK. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia 2006;77:511-4.
22. Foo LY. Amarulone, a novel cyclic hydrolysable tannin from Phyllanthus amarus. Nat Prod Lett 1993;3:45 - 52.
23. Glick Z, Joslyn MA. Food intake depression and other metabolic effects of tannic acid in the rat. J Nutr 1970;100:509-15.
24. Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37-46.
25. Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, Oral U. Liver and kidney toxicity in chronic use of opioids: An experimental long term treatment model. J Biosci 2005;30:245-52.
26. Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 2004;37:287-303.
Correspondence:
Dr Uba Nwose.
SWPS 590 Smollett Street.
Albury NSW 2640, Australia.
E-Mail: ezekiel.nwose @ gsahs.health.nsw.gov.au
