Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
Letters to the Editor / Cartas al Editor
THE USE OF ELEVATED PLUS MAZE TO STUDY THE EFFECTS OF AQUEOUS EXTRACT OF GARCINIA KOLA (Linn) ON THE ANXIETY STATUS OF MALNOURISHED MICE
Sunday A. Ajayi1, Nwoha P.U.2
1 Department of Anatomy, College of Medicine. University of Ado-Ekiti, Ado-Ekiti. Ekiti state.
2Department of Anatomy and Cell Biology, Faculty of Basic Medical Sciences. Obafemi Awolowo University, Ile-Ife.
Nigeria
E-mail: bravodesunny @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2011;2:63-67.
To the Editor:
Garcinia kola seeds are chewed as a masticatory substance to stimulate the flow of saliva, and widely consumed as snack1. Unlike other kola nuts (Kola nitida, Kola acuminata), Garcinia kola is thought to clean the digestive system, without abdominal problems, even when a lot of the nuts are eaten1. Garcinia kola is culturally very important for the Yoruba and Igbo tribes of Nigeria and for many other people living in the sub-saharan Africa. For centuries the nuts have been an important part of their lives from birth to death. They are used in traditional ceremonies, marking special events like births, marriages and conferring chieftaincy titles. A Garcinia kola nut tree may be planted when a baby is born with the child becoming its life long owner. In proposals of marriage, young men offer Garcinia kola nuts to the father of the bride, and an exchange of Garcinia kola nuts is essential in many business dealings as well2.
The quest for naturally occurring compounds of herbal or plant origin that could be of benefit as contraceptive and fertility control agents stimulated the interest of Isawumi3 in Garcinia kola, seeds which are widely consumed as a stimulant. The traditional African medicinal uses include treatment of cough, purgative, anti-parasitic and anti-microbial4,5. The seed is used in the treatment of diarrhoea6 bronchitis and throat infections7,8 and liver disorder9. The Garcinia kola seeds enjoy a folk reputation in Africa as a poison antidote10. In addition, the plant possesses anti-hepatotoxic11,12, antioxidant13, hypoglycemia9,14 and aphrodisiac properties15. Garcinia kola seeds have been reported to have an anti-inflammatory activity4,16. These studies have revealed that the process of ovulation is comparable to an inflammatory process17. Anti-inflammatory drugs have been employed in blocking ovulation18.
Some flavonoids (including apigenin based) suppress the formation of cyclo-oxygenase-2 enzyme thus playing an important role in the prevention of cancer and inflammation, partly via inhibiting cox-2 enzymes. This property is also currently under trial in chemoprevention potentials against human cancers as many types of cancer cells use cox-2 to propagate17.
Garcinia kola seeds contain biflavonoid capable of having anti-inflammatory properties11 and are a natural antioxidant13,19. Constituents of the seed of Garcinia kola include 1 - 3, 8 - 11 benzophenones, Garcinia biflavonones (GB-1, GB- 2) and kola flavonone20. Apigenin based flavonoids represent 60% of the total flavonoids present in the diethyl ether fraction of Garcinia kola seed21. Phenolic compounds likely to be present in Garcinia kola are mostly secondary plant metabolites like tannin, saponin, oxalates etc. High consumption of these compounds is therefore dangerous to health. In conclusion, pharmacologic importance of Garcinia kola cannot be ruled out all the same high quantity of Garcinia kola should not be consumed at a time considering the adverse effects of high saponin, cyanogenic glycoside and other glycoside20.
Malnutrition is a general term for the medical condition caused by an improper or insufficient diet. It most often refers to undernutrition resulting from inadequate consumption, poor absorption, or excessive loss of nutrients, but the term can also encompass overnutrition, resulting from over eating or excessive intake of specific nutrients. An individual will experience malnutrition if the appropriate amount of, or quality of nutrients comprising of healthy diet are not consumed for an extended period of time. An extended period of malnutrition can result in starvation22. Malnutrition, as the lack of sufficient nutrients to maintain healthy bodily functions, is typically associated with extreme poverty in economically developing countries. It is a common cause of reduced intelligence in parts of the world affected by famine such as Ethiopia23. Malnutrition as the result of inappropriate dieting, overeating or the absence of a "balance diet" is often observed in economically developed countries (e.g. as indicated by increasing levels of obesity). Most commonly, malnourished people either do not have enough calories in their diet, or are eating a diet that lacks protein, vitamins, or trace minerals. Medical problems arising from malnutrition are commonly referred to as deficiency diseases. Scurvy is a well-known and now rare form of malnutrition, in which the victim lacks vitamin C.
Thirty-two adult mice with average weight of 20g were used. They were bred in the Animal Holdings of the Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ile-Ife, Nigeria. Four plastic cages were procured for the four different groups of the animals. Proper care of the animals was based on Leader24 and the National Institute of Health (U.S. PHS policy) on humane care of laboratory animal25.
The malnourished feed with which the animals were fed was prepared in the Histology Laboratory of the Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ile-Ife. The formulation of the feed is according to Robinson26 and Nwoha27 as stated: Grains 72g, Casein 3g, Cellulose 8g, Oil 6g, Sucrose 5g, Vitamin 6g. Accurate measurements of the parameters stated above was ensured with the use of Mettler Toledo sensitive balance. They were mixed together with water, molded and oven dried before feeding the animals with it. Garcinia kola nuts were bought from the local market in Ile-Ife, Nigeria. The outer coats were removed, and the seeds were cut into pieces and air-dried. The dried-seeds were ground into fine powder and cold extraction was done using distilled water in a soxhlet extraction kit at the Science Central Laboratory of the Obafemi Awolowo University, Ile-Ife, Nigeria. 20 g of the extract was weighed out and dissolved in 100 ml of distilled water to give 200 mg/ml of aqueous extract of Garcinia kola.
3-Nitropropioic acid was obtained from Sigma Chemical Co. St. Louis, U.S.A. The mice in the treatment groups received 20 mg/kg body weight of 3-Nitropropioic acid intraperitoneally. The control groups received an equivalent volume of distilled water intraperitoneally. After administration the mice were left in their cages for the next three days to allow for the neurotoxic effects of the drug on the hippocampus28.
The mice were carried to the test room in their home cages and tested one at a time for 5 minutes each. Mice were handled by the end of their tails at all times. They were taken from their home cages and placed randomly into the apparatus for exploration. After the 5 minutes test, the mice were returned to their cages and the apparatus was cleaned with 70% ethanol and permitted to dry between tests.
Elevated plus maze was used to determine the anxiety level of the mice. This apparatus consists of two open arms and two arms that are enclosed by high walls. The open arms are perpendicular to the closed arms, with the four arms intersecting to form the shape of a plus sign. The elevated plus-maze is usually elevated approximately 50cm above the floor. Security is provided by the closed arms while the open arms offer exploitatory value. Therefore, one might expect anxious mice to spend less time in the open arms than those that are less fearful29. The mice were placed in the centre of the elevated plus-maze and activities are recorded for 5 minutes.
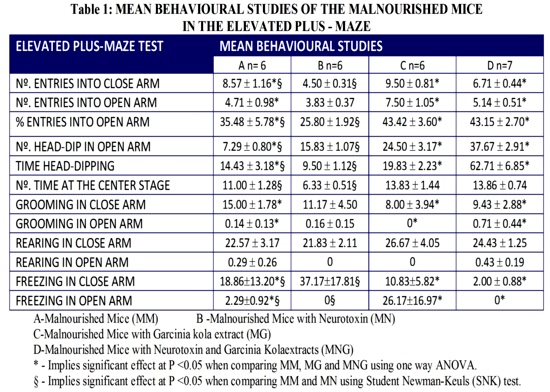
With regard to the behaviour of MM, MG and MGN, one way ANOVA revealed that there was significant effect of intake of Garcinia kola on number of entries into close arm (F2, 16 = 5.30; P<0.05); number of entries into open arm (F2, 16 = 5.31; P<0.05) ; % entries into open arm (F2, 16 = 7.82; P<0.05); number of head-dip in open arm, (F2, 16 = 32.08; P<0.05); time head-dipping (F2, 16 = 29.70; P<0.05); grooming in close arm (F2, 16 = 12.29; P<0.05), grooming in open arm (F2, 16 = 11.93; P<0.05); freezing in close arm, (F2, 16 = 23.01; P<0.05); freezing in open arm (F2, 16 = 13.14; P<0.05), but has no significant effect on number of entries at the center stage (F2, 16 = 0.93; P<0.05) rearing in close arm (F2, 16 = 0.40; P<0.05), rearing in open arm (F2, 16 = 0.74; P<0.05). Going by the (SNK) test, there were significant effects on number of entries into close arm, % entries in open arm, number of head-dip in open arm, Time head-dipping, number of time at the center stage, freezing in close arm and freezing in open arm, but not significant on number of entries into open arm, grooming in open arm, grooming in close arm, rearing in open arm and rearing in close arm (P<0.05) when comparing MM, and MN (Table 1).
The elevated plus maze has been described as a simple method for assessing anxiety responses of rodents30. A task, using a Y-shaped apparatus that included an elevated open alley, which produced a strong approach-avoidance conflict, and an enclosed alley, which did not29. This task was modified into an elevated maze with four arms (two open and two enclosed) that are arranged to form a plus shape and was described by Handley and Mithani331. These authors described the assessment of anxiety behavior of rodents by using the ratio of time spent on the open arms to the time spent on the closed arms. Unlike other behavioral assays used to assess anxiety responses that rely upon the presentation of noxious stimuli (i.e., electric shock, food/water deprivation, loud noises, exposure to predator odor, etc.) that typically produce a conditioned response, the elevated plus maze relies upon rodents' proclivity toward dark, enclosed spaces (approach) and an unconditioned fear of heights/open spaces32. There is great diversity in possible applications of the elevated plus maze. To name a few, prescreening of newly developed pharmacological agents for treatment of anxiety-related disorders can be carried out. The anxiolytic and anxiogenic effects of pharmacological agents, drugs of abuse and hormones can be investigated. The effects of reproductive senescence/aging and/or pre-, peri- or postnatal exposure to various stressors can be assessed. Furthermore, beyond its utility as a model to detect anxiolytic effects of benzodiazepine-related compounds, the elevated plus maze can be used as a behavioral assay to study the brain sites (e.g., limbic regions, hippocampus, amygdala, dorsal raphe nucleus, etc.)33,34 and mechanisms (e.g., GABA, glutamate, serotonin, hypothalamic-pituitary-adrenal axis neuromodulators, etc.)35 underlying anxiety behavior. Indeed, the elevated plus maze has been used as a model of state, unconditioned anxiety for over two decades.
Behavioral responses in the elevated plus maze are easily assessed and quantified by an observer. Briefly, rodents are placed in the intersection of the four arms of the elevated plus maze and their behavior is typically recorded for 5 min. This was based upon the early studies by Montgomery29 that revealed that rats demonstrated the most robust avoidance responses in the first 5 min after placement in the elevated open alleys. The behaviors that are typically recorded when rodents are in the elevated plus maze are the time spent and entries made on the open and closed arms. Behavior in this task (i.e., activity in the open arms) reflects a conflict between the rodent's preference for protected areas (e.g., closed arms) and their innate motivation to explore novel environments. Anti-anxiety behavior (increased open arm time and/or open arm entries) can be determined simultaneously with a measure of spontaneous motor activity (total and/or closed arm entries), albeit the arm entries made in the maze may not be an optimal measure of motor activity. Other ethological measures that can be observed in rodents in the maze are the number of rears, head dips, fecal boli, freezing or stretched-attend postures.
In the elevated plus-maze, % entries into open arm was greatest in MG, followed by MNG, then by MM, and least in MN. The % open arm entries and % time spent in open arms are both measures of fear and anxiety30,36. This showed that MN were the most fearful and demonstrated greatest level of anxiety. They have least head-dips and greatest grooming which showed that they were least active. Freezing in both close and open arm were greatest in these mice which showed an evidence of being fearful, and rearing both in close and open was least in them that indicated least locomotor activity.
Considering all the behavioural activities, it could be deduced that MN were most fearful, dormant, anxious, less active, with low exploratory activity but were protected from all these by feeding with aqeous extract of Garcinia kola.
REFERENCES
-
1.- Adjanohun E et al. Traditional Medicine and Pharmacopoeia: contribution to ethnobotanical and floristic studies in Western Nigeria OAU/STRC. 1991
2. Atilade Akanmu Adebisi. A case study of Garcinia kola nut production - to - consumption system in J4 Area of Omo forest reserve, south west Nigeria Pg. 2000:1-18.
3.- Isawumi AM. The Common edible fruits of Nigeria, Part II. The Nigerian field 1993;58:1-2.
4.- Madubunyi II. Antimicrobial activities of the constituents of Garcinia kola seeds. Intern J Pharmacog 1995; 33:233-237.
5.- Okunji CO, Iwu MM. Molluscidal activity of Garcinia kola biflavanones. Fitoterapia. 1991; 62:74-76.
6.- Braide VP. Pharmacological effect of chronic ingestion of Garcinia kola seeds in rats. Phytotherapy Res 1991; 4:39-41
7.- Orie NN, Ekon EU. The bronchodilator effect of Garcinia kola East Afr Med J. 1993; 70(3): 143-145.
8.- Adebisi AA. Marketing and Post-harvest constraints of the African star apple (Agbalumo). In Ladipo DO, and Denton AO, (eds) "Processing on the potential and conservation of chrysophyllum albidum in Nigeria". NIHORT/ NACGRAB/ CENRAD. 1997.
9.- Iwu MM, Igboko OA, Elekwa OK, Tempesta MS. 1990 Prevention of thioacetamide-induced hepatotoxicity by biflavanones of Garcinia kola. Phytother Res. 1990;4. 157-159.
10.- Agboola DA, Adedire MO. Response of treated dormant seeds of three tropical tree species to germination promoters. Nigerian J Botany. 1998;11:103-110
11.- Akintowa A, Essien A. Protective effects of Garcinia Kola seed extracts against paracetamol induced hepatotoxicity in rats. J Ethnopharmacol. 1990;29:207-211.
12.- Braide VP. Antihepatotoxic Biochemical effects of Kolaviron, a biflavonoid of Garcinia Kola seeds. Phytotherapy Res 1991; 5:35-37.
13.- Farombi EO, Akanni OO, Emerole GO. Antioxidant and scavenging activity of flavonoid extract (Kolaviron) of Garcinia kola seeds. Pharm Boil 2002;40: 107-116.
14.- Adegoke GO, Kumar MV, Sambaiah K, Lokesh BR. Inhibitory effect of Garcinia kola on Lipid Peroxidation in rat liver homogenate. Indian J Exp Biol. 1998;36: 907-910.
15. Ajibola AO Satake M. Contributions to the phytochemistry of medicinal plants growing in Nigeria as reported in the 1979-1990 literature - A preview. Afr J Pharm Sci 1992; 22:172-201.
16.- Braide VP. Anti-inflammatory effect of kolaviron, a biflavonoid extract of Garcinia Kola. Fitoterapia 1993; LXIV:433-436
17.- Gonzalez FJ, Gelboin HV. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev. 1994;26(1-2):165-83.
18.- Kadlubar FF, Hammons GJ. The role of Cytochrome P450 in the metabolism of chemical carcinogens. In: Guengerich FP (Ed). Mammalian Cytochrome P450. CRC Press, Boca Raton, FL. 1987; I:81-130.
19.- Terashima K, Takaya Y, Niwa M. Powerful antioxidative agents based on garcinoic acid from Garcinia kola. Bioorg Med Chem. 2002;10:1619-1625.
20.- Cotterih P, Scheinmenn F, Stenhuise I Composition of Garcinia kola seeds J Chem Soc Perkin Trans. 1978; 1:532-533.
21.- Iwu MM, Igboko O. Flavonoids of Garcinia kola seeds. J Natural Prod. 1982; 45:650-651.
22.- Nielsen R. The little green handbook. Picador, New York 2006: 23-24.
23.- Wine M. Malnutrition is cheating its survivors, and Africa's Future. The New York Times; December 28, 2006.
24.- Leader RW, Stark D. The Importance of Animals in Biomedical Research. Perspective Biol. 1987;30: 470-485.
25.- National Institutes of Health. OPRR Public Health Service Policy on Humane Care and Use of Laboratory Animals. US Department of Health and Human Services. Office for protection from Research Risk. Rockville, MD. 1996
26.- Robinson PH, Udén P, Wiseman J, Mateos GG. Some suggestions and guidelines for preparation of manuscripts for submission for consideration for publication, Animal Feed Sci Technol 2007; 134: 181-188.
27.- Nwoha PU, Ayoka A, Ibeh J, Okoro I. J Environ Neurosci Biomed 2007; 1: 26-32.
28.- Acevedo-Torres K, Berríos L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington's disease. DNA Repair (Amst). 2009 1;8:126-136.
29.- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254-260.
30.- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985:14:149-167.
31.- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of 'fear'-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1-5.
32.- Barnett SA. The Rat-A Study in Behavior. Ed. Univ. Chicago Press. Chicago, 1975.
33.- Gonzalez LE, File SE. A five minute experience in the elevated plus-maze alters the state of the benzodiazepine receptor in the dorsal raphe nucleus. J Neurosci. 1997;17:1505-1511.
34.- Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res. 1993;56:115-118.
35.- Overstreet DH, Commissaris RC, De La Garza R 2nd, File SE, Knapp DJ, Seiden LS. Involvement of 5-HT1A receptors in animal tests of anxiety and depression: evidence from genetic models. Stress. 2003;6:101-110.
36.- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987; 92: 180-185.
CORRESPONDENCE:
Sunday A. Ajayi,
University of Ado-Ekiti,
College of Medicine, Department of Anatomy,
Ado-Ekiti, Ekiti State.
Nigeria.
E-mail: bravodesunny @ yahoo.com
Received: July 20, 2011.
Published: September 30, 2011
