Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
Letters to the Editor / Cartas al Editor
RENAL FUNCTIONAL EQUATIONS: THEIR EVOLUTION AND ROLE IN CKD PATIENTS
Joaquín Álvarez Gregori MD. PhD.1, Carlos G. Musso MD.PhD.2,
José R. Jauregui MD.PhD.2; Juan F. Macías Núñez MD. PhD.1
1Universidad de Salamanca. Facultad de Medicina. Salamanca. Spain.
2Unidad de Biología del Envejecimiento. Hospital Italiano de Buenos Aires - Argentina.
carlos.musso @ hospitalitaliano.org.ar
Rev Electron Biomed / Electron J Biomed 2013;1:56-61.
To the Editor:
Traditionally, glomerular filtration rate (GFR) has been identified as the best marker of global renal function, calculated by using substance clearance techniques such as inulin or creatinine aided with cimetidine1
Chronic kidney disease (CKD) is a syndrome which derives from a progressive and generalized deterioration of renal function secondary to nephronal mass destruction, and renal functional evaluation is very important for doing its diagnosis and follow up2,3. However, since all clearance techniques have some degree of difficulty, an easier way of determining GFR, equations to estimate glomerular filtration have been developed. In the present review article we analyzed which was the evolution of these renal functional equations, and which is their role in CKD patients.
In order to have an easier way of determining GFR, equations to estimate glomerular filtration have been developed, most of them mainly based on serum creatinine (Table 1).
Kampmann et al.4, Cockcroft and Gault5 and Rowe et al.6 described renal function formulas for estimating GFR in the clinical practice. Cockcroft and Gault's formula (1976) is the most frequently used, although it has been questioned due to the fact that it exaggerates the decline in GFR, at least in people older than 804-7. However, Nicoll et al. found a good correlation in 18 individuals of ages between 66 and 82 using eGFR calculated according to Cockcroft and Gault's formula and the one obtained with 99Tc-DTPAm7.
One of the problems that appeared when interpreting such studies was that they did not use individuals who represented the population well. Rowe et al. examined healthy old people in the community, while Kampmann et al.4 used hospital population excluding those patients with high levels of creatinine in blood in comparison with healthy adults. Cockcroft and Gault5 used hospitalized patients for their study, without excluding anyone regardless of their renal function3-5.
In 1987 Keller8 pointed out that the simplest formula to estimate GFR for people between 25 and 100, with normal creatinine values, is: [130-age (in years) ml/min]. In the last 20 years other formulas have been developed to predict glomerular filtration using indirect calculations and serum creatinine as a starting point, such as Nankivell's9, and Baracskay's10 (Table 1)7-10.
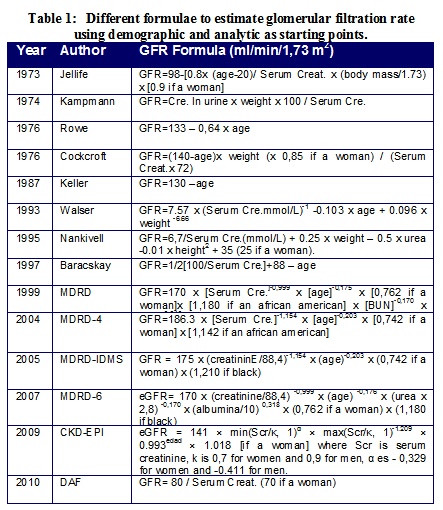
In 1999, with the aim of being more precise regarding glomerular filtration, the MDRD group (The Modification of Diet in Renal Disease) published a new equation to estimate GFR based on creatinine clearance and the concentration of serum creatinine taking into account the demographic and clinical characteristics in patients previously diagnosed with CKD. However, this equation has not been proven in people without renal disease, people with type 1 and 2 diabetes in treatment with insulin, people younger than 18, old people (older than 70), pregnant women, patients with comorbidities and transplant recipients11.
In 2001 Lewis et al.12 recalculated the formula, adding renal transplanted and Afro-American patients with nephrosclerosis. However, neither of the formulas were applied to subgroups: healthy, diabetic and people older than 70. Therefore, such equations are not valid for the general population. Despite all these findings, patients who have a moderate GFR reduction between 30 and 59 ml/min/1.73 m2, are still considered in the CKD threshold. If we take into consideration this criteria for diagnosing CKD, by eGFR < 60 ml/min/1.73 m2, it would incorrectly indicate that approximately 17% of people older than 60 would suffer from CKD 11-13.
In 2009 the CKD-EPI formula was created with the aim of obtaining more reliability for the calculation of eGFR based on the levels of creatinine in blood, but despite the fact that it is more reliable and accurate than MDRD, it appears to have important limitations regarding the representation of the population and, particularly, since it does not have a significant sample of people older than 70 years14.
In any case, when we use the formulas or tests based on serum creatinine values we should take into account that such values per se are not an optimal marker of GFR. There are well documented data which point to the fact that serum creatinine values can vary significantly in multiple scenarios such as the patient´s metabolic state, their muscle mass, states of hyper or dehydration, some medication (cimetidine) and tubular handling (creatinine backfiltration). All these factors could cause errors in those formulas which use the concentration of serum creatinine to estimate GFR14-16.
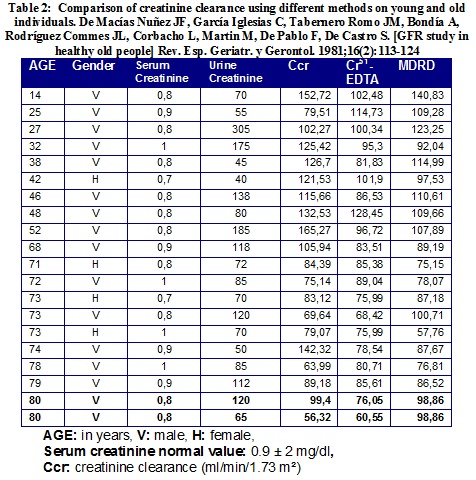
As we can appreciate in Table 2, there are significant differences in a GFR value when it is obtained using creatinine clearance, Cr51-EDTA and the MDRD formula. It can be identified, at the end of the table, that two 80 year old males with the same serum creatinine have substantially different glomerular filtration rates depending on the method used. As both men are the same age and have the same serum creatinine, they have the same GFR value calculated with the MDRD (98.8 ml/min/1.73m2) formula. If we use creatinine clearance instead of MDRD, one of them has a GFR of 99 ml/min/1.73m2, while the other only reaches a value of 56.3 ml/min/1.73m2.
It is worth noting that the difference between these two healthy old men is in the elimination of urinary creatinine: 120 mg/dL in one and 65 mg/dl in the other. This phenomenon could be explained by creatinine backfiltration phenomenon already described in aged people. It is also interesting to observe that both of them have a comparable GFR value (76 and 60 ml/min/1.73 m2) when Cr51-EDTA is used. As a result the same person may be considered as affected with CKD or not depending on the method used to estimate GFR.
There are many difficulties regarding the recommendation of basing CKD diagnosis just on a eGFR critical value, not taking into account other variables such as age, gender, race, renal disease etiology, and associated pathologies17, 18.
For instance, in stage 3 - CKD (GFR between 30-60 ml/min/1.73 m²), even though a diagnosis has been established by documenting eGFR < 60 ml/min/1,73m2 during a period longer than three months, it should be pointed out that this criteria does not necessarily apply to elderly people since GFR reduction can present as a consequence of normal ageing17, 19.
Similarly, a petit vegetarian woman with eGFR < 60 ml/min/1.73 m2, who has a very positive renal reserve (> 100 %), and is not suffering from any of the classically associated complications to CKD such as uremic symptoms, anemia, hyperphosphatemia, hypocalcemia, metabolic acidosis, hyperparathyroidism, altered urinalysis, and/or abnormal renal ultrasound, should not be considered a CKD patient14, 20
Even more, some authors do not support the idea of a eGFR < 60 ml/min/1.73 m2 "critical value" as an independent risk factor to develop CKD in the future. Firstly, according to what was published by Go et al., independent mortality factors do not increase with eGFR values between 45 and 59 ml/min/1.73 m2 when chronic damage has been established from serial measurements of serum creatinine21. Secondly, a decrease in mortality risk in people older than 45 has been demonstrated, with a GFR between 50 to 59 ml/min/1.73 m2 when chronic damage is established in a period of 3 to 6 months22,23. Thirdly, the PREVEND study shows that approximately two thirds of the patients in stage 3 - CKD do not present albuminuria and their risk of cardiovascular complications, according to the tables adjusted by age and gender, were similar to those people who did not present renal disease23.
Another problem related with performing CKD diagnosis based on eGFR is that the obtained CKD prevalence data is exceedingly variable depending on the applied formula24-27. In this sense, the EPIRCE study (2010) found a global prevalence of CKD in stages 3 to 5 (according to the NKF-K/DOQI recommendations with eGFR < 60 ml/min/1.73 m2) of 6,8%, increasing this number to 21,4% in people older than 6424. In the EROCAP study (2007), the prevalence of CKD was studied with the same criteria of eGFR < 60 ml/min/1.73 m2 obtained in 9233 patients older than 18 who attended a primary health care consultation. According to its results, global prevalence varied depending on the eGFR formula used, between 21,3% and 22.7% while in the population older than 70 it reached 33,7%28,29.
In 2008 Zhang and Rothenbacher conducted a systematic review of 26 studies on the prevalence of CKD in different geographical areas of the world29. Respecting the same estimation criteria as glomerular filtration, the CKD diagnosis and values which were < 60 ml/min/1.73 m2, resulting in a global media prevalence in the adult population older than 30 of 7,2% while in people 64 or older it varied between 23,4% and 35,8%29,30. Then, it seems that eGFR formulas are much more helpful in CKD staging and follow up, than in its diagnosis.
In order to avoid diagnostic errors like the above mentioned one, a new formula has been developed for diagnosing CKD: HUGE formula. It does not take into account patient´s eGFR for diagnosing CKD but two biochemical variables, and a clinical one: hematocrit, uremia, and gender. This formula is as follows
HUGE = 2.505458 - (0.264418 x Hematocrit) + (0.118100 x Urea) [+ 1.383960 if male], where a value > 0 diagnoses CKD.
HUGE formula allows for the discrimination between a healthy old person (HUGE<0) and a CKD patient (HUGE>0), both with similar eGFR, with high sensitivity and specificity, especially in people older than 7031,32.
In conclusion, according to the aforementioned considerations, we should state that glomerular filtration estimations, in particular those obtained with the MDRD formula or the CKD-EPI formula are, undoubtedly, valid to stage and follow up on the progress of patients already diagnosed with CKD. However, the use of eGFR lower than 60 ml/min/1.73 m2 to follow up on patients without a known diagnosis is not only controversial but also perhaps not recommended.
On the other hand, to establish an incorrect diagnosis of CKD using estimations of GFR which are lower than 60 ml/min/1.73 m2 obtained through routine lab tests could be considered arbitrary, insufficient and especially inadequate in the old population (older than 70).
Conflict of interests: The authors declare not to have conflict of interests in this study
REFERENCES
1) Rennke H, Denker B. Renal Pathophysiology. Philadelphia. Lippincott Williams & Wilkins. 1994: 267-290
2.- Clarkson M, Magee C, Brenner B. Approach to the patient with renal disease. In The kidney. Philadelphia. Saunders. 2010: 3-41
3.- Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT; PREVEND Study Group. Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008; 23(12): 3851-3858.
4.- Kampmann J, Siersbaek-Nielsen K, Kristensen M, Hansen JM. Rapid evaluation of creatinine clearance. Acta Med Scand. 1974; 196(6): 517-520.
5.- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron, 1976; 16(1): 31-41.
6.- Rowe JW, Shock NW, DeFronzo RA. The influence of age on the renal response to water deprivation in man. Nephron. 1976; 17(4): 270-278.
7.- Nicoll SR, Sainsbury R, Bailey RR, King A, Frampton C, Elliot JR, Turner JG. Assessment of creatinine clearance in healthy subjects over 65 years of age. Nephron. 1991; 59(4): 621-625.
8.- Keller F. Kidney function and age. Nephrol Dial Transplant. 1987;2(5):382.
9.- Nankivell BJ, Chapman JR, Allen RD. Predicting glomerular filtration rate after simultaneous pancreas and kidney transplantation. Clin Transplant. 1995;9(2):129-134.
10.- Baracskay D, Jarjoura D, Cugino A, Blend D, Rutecki GW, Whittier FC. Geriatric renal function: estimating glomerular filtration in an ambulatory elderly population. Clin Nephrol. 1997;47(4): 222-228.
11.- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130(6): 461-470.
12.- Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O'Connor D, Ojo A, Phillips R, Sika M, Wright J Jr; African-American Study of Hypertension and Kidney Disease. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001; 38(4): 744-753.
13.- Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000; 11: 155A.
14.- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003; 41(1): 1-12.
15.- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
16.- Musso CG, Michelángelo H, Vilas M, Martinez B, Bonetto A, Jauregui R, Algranati L. Renal creatinine handling in very old patients with chronic renal disease. Int Urol Nephrol. 2011;43(3):899-902.
17.- Musso CG, Macías Núñez JF, Oreopoulos D. Physiological similaritis and differencies between renal ageing and chronic renal disease. J Nephrol. 2007; 20: 586-587
18.- Xie P, Huang JM, Lin HY, Wu WJ, Pan LP. CDK-EPI equation may be the most proper formula based on creatinine in determining glomerular filtration rate in Chinese patients with chronic kidney disease. Int Urol Nephrol. 2012 Nov 8. [Epub ahead of print]
19.- Musso CG, Oreopoulos D. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119 Suppl 1:p1-5.
20.- Musso CG. Renal reserve test: its methodology and significance. Saudi J Kidney Dis Transpl. 2011;22(5):990-993.
21.- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351(13): 1296-1305.
22.- Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324.
23.- Clase CM, Kiberd BA, Garg AX. Relationship between glomerular filtration rate and the prevalence of metabolic abnormalities: results from the Third National Health and Nutrition Examination Survey (NHANES III). Nephron Clin Pract. 2007;105(4):c178-184.
24.- O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758-2765.
25.- O'Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC. Mortality risk stratification in chronic kidney disease: one size for all ages?. J Am Soc Nephrol. 2006;17:846-853.
26.- Otero A, de Francisco A, Gayoso P, García F; EPIRCE Study Group. Prevalence of chronic renal disease in Spain: results of the EPIRCE study. Nefrologia. 2010;30:78-86.
27.- de Francisco AL, De la Cruz JJ, Cases A, de la Figuera M, Egocheaga MI, Górriz JI, Llisterri JI, Marín R, Martínez Castelao A. Prevalencia de insuficiencia renal en Centros de Atención Primaria en España: Estudio EROCAP. Nefrologia 2007; 27:300-312.
28.- Robles NR, Felix FJ, Fernandez-Berges D, Perez-Castán JF, Zaro MJ, Lozano L, Alvarez-Palacios P, Garcia-Trigo A, Tejero V, Morcillo Y, Hidalgo AB. Cross-sectional survey of the prevalence of reduced estimated glomerular filtration rate, albuminuria and cardiovascular risk in a native Spanish population. J Nephrol. 2013;26:675-682.
29.- Libório A, Uchoa R, Neto J, Valdivia J, Daher Ede F, Mejia J. Assessing glomerular filtration rate in patients with severe heart failure: comparison between creatinine-based formulas. Sao Paulo Med J. 2012;130:289-293.
30.- Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117
31.- Alvarez-Gregori JA, Robles NR, Mena C, Ardanuy R, Jauregui R, Macas-Nu Nunez JF. The value of a formula including haematocrit, blood urea and gender (HUGE) as a screening test for chronic renal insufficiency. J Nutr Health Aging. 2011;15:480-484
32.- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med. 2003;163: 356-360.
CORRESPONDENCE:
Dr. Carlos Musso PhD.
Hospital Italiano de Buenos Aires
Perón 4190.
Buenos Aires. Argentina
E-mail address: carlos.musso @ hospitalitaliano.org.ar
Received: July 7, 2013.
Published: July 16, 2013
