Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
- quinolone resistance in E. coli in urine cultures in outpatients according to data obtained in the 1995-1996 Red WHONET: 2%.
- Resistance in urine cultures in patients admitted through the Central Emergency Hospital Italiano de Buenos Aires, in the first half of 2010: 23-50% (23% are male and female patients <60 years and 50% in men> 60 years without hospitalization).
EVALUATION OF CYSTICLEAN® CAPSULES, A CRANBERRY EXTRACT WITH HIGH ANTI-ADHESION ACTIVITY, AS MONOTHERAPY IN UNCOMPLICATED CYSTITIS: AN OBSERVATIONAL PILOT STUDY
Ester Risco Rodríguez Pharm. PhD.1,
Humberto Suárez MD. PhD.2,
Isidre Bonet MD. PhD.3, Jesús-José Cuadrado Blanco MD. PhD.4
1Phytonexus, S.L. Valencia. 2Instituto Médico Tecnológico, 3Teknon Clinic, Urology Department. Barcelona.
4GerHogar. Urinary Incontinence Unit. Salamanca. Spain
ester.risco @ phytonexus.com
Rev Electron Biomed / Electron J Biomed 2015;2:19-28
Comment of the reviewer Dra. Ines Staneloni. Infectious Diseases Section of Internal Medicine Division, and Coordinator of Infection Prevention Committee. Hospital Italiano de Buenos Aires, Argentina.
Comment of the reviewer Dr. Astrid Smud. nfectious Diseases Section of Internal Medicine Division, Hospital Italiano de Buenos Aires, Argentina.
RESUMEN:
Introducción: Cysticlean® es un producto de extracto de arándano con una alta cantidad de proantocianidinas (240 mg / cápsula) con una actividad anti-adhesión dependiente de la dosis significativa de Escherichia coli (EC) adherida a las células uroepiteliales. Ensayos clínicos previos mostraron que Cysticlean® es un producto altamente recomendado en la profilaxis y tratamiento de las infecciones urinarias. El objetivo de este estudio es la evaluación de Cysticlean como una alternativa a los antibióticos para tratar la cistitis no complicada.
Material y Métodos: Este estudio observacional incluyó a 30 pacientes consecutivos ambulatorios (17 mujeres y 13 hombres), que fueron diagnosticadas de cistitis no complicada (CNC) y aceptaron participar en este estudio observacional. Los pacientes fueron citados nuevamente a control médico después de 15 días de iniciado el tratamiento con Cysticlean® (1 cápsula de Cysticlean® cada 12 h al día) o de inmediato si los signos / síntomas no habían desaparecido. En este caso, Cysticlean® fue suspendido y los pacientes fueron tratados con antibióticos.
Resultados: Se observó que 21 pacientes se curaron exitosamente empleando solamente el tratamiento a base de Cysticlean® (70%), mientras que en 9 pacientes fue necesario el uso de antibiótico para curar su CNC. 82,35% de las mujeres y 53, 85% de los hombres no necesitaron antibióticos para curarse. Además no se observaron diferencias significativas, al inicio del estudio, en los signos / síntomas de gravedad entre los pacientes curados solo con extracto y aquellos que necesitaron antibióticos. Tampoco se informaron efectos secundarios ni reacciones adversas al producto.
Conclusiones: Estos datos preliminares sugieren que Cysticlean® podría ser considerado como una alternativa a los antibióticos para el tratamiento de CNC, aunque se requieren aun otros estudios clínicos para confirmar si Cysticlean® podría ser una alternativa al tratamiento con antibióticos de la CNC.
PALABRAS CLAVE: Arándano. Vaccinium macrocarpon. Cysticlean®. Proantocianidinas. Cistitis no complicada. Infecciones del tracto urinario.
SUMMARY:
Background:Cysticlean® is a cranberry extract product with a high quantity of proanthocyanidins (240 mg/capsule) with a significant dose-dependent anti-adhesion activity of Escherichia coli (EC) adhered to uroepithelial cells. Previous clinical assays showed that Cysticlean® is a product highly recommended in the prophylaxis and treatment of UTIs. The aim of this study is the evaluation of Cysticlean as an alternative to antibiotics to treat uncomplicated cystitis.
Material and Methods This observational study included 30 consecutive ambulatory patients (17 women and 13 men), who were diagnosed of uncomplicated cystitis (UC) and agreed to participate in this observational study. Patients were informed to come to visit the doctor again after 15 days after Cysticlean® treatment was started (1 capsule of Cysticlean® every 12 h daily) and immediately if signs/symptoms did not disappear. In this case, Cysticlean® was stopped and patients treated with antibiotic.
Results: 21 patients were successfully cured with Cysticlean® treatment only (70%) and 9 patients needed antibiotic to cure their UC. 82.35% of women and 53.,85% of men did not need antibiotic to be cured. No significant differences at baseline were found regarding signs/symptoms severity between those patients cured with extract alone and those who needed antibiotic. No side effects/adverse reactions were reported.
Conclusions: These preliminary data strongly suggest that Cysticlean® could be considered as an alternative to antibiotics for a 1st line treatment of UC. Further clinical studies to confirm whether Cysticlean® could be an alternative to antibiotic treatment for UC and this approach could contribute to reduce world-wide growing antibiotic resistance.
KEY WORDS: Cranberry. Vaccinium macrocarpon. Cysticlean®, Proanthocyanidins. Uncomplicated cystitis. Urinary tract infections. UTIs.
INTRODUCTION
Uncomplicated cystitis (UC) is the most common urinary tract infections (UTIs) in the world, mainly affecting women and elderly men1, and it is a symptomatic cystitis without fever and general discomfort. Among risk factors associate to UC are: lack of physical activity, history of recurrent cystitis (more than 3 or 4 per year), sedentary, urinary retention, insufficient water ingestion, obesity, hypotonic pelvic floor and prostatitis. There are also other unknown factors (genetics) and often it is possible to find a combination of described risk factors in patients with UC.
Escherichia coli (Ec) is the most frequent causative pathogen of this infection2-3. The diagnosis of UTI refers to the presence of clinical signs and symptoms arising from the genitourinary tract plus the presence of one or more microorganisms in the urine exceeding a threshold value for significance.
Acute antibiotic treatments are the conventional therapy, used successfully in various regimens, to treat UC. Several studies reported that these antibiotic treatments were effective in more than 90% of these patients; however, the prevalence rates of antibiotic resistance among Ec and other uropathogens have significantly increased4-7. Depending on the most common antibiotic used highest is the incidence of antibiotic resistance to this antibiotic. Nowadays it has been found that E. coli shows an antibiotic resistance higher than 50% against to the most common antibiotics prescribed to treat UC (i.e. ciprofloxacin, fosfomycin or trimethoprim/sulfamethoxazole). In these cases, several other antibiotics are needed to successfully treat UC, increasing the risk to increase resistance. Antibiotic resistance, which has traditionally been a problem only in nosocomial complicated UTI, is also now becoming a major risk in uncomplicated community-acquired UTIs8. Therefore, UC treatment should be a serious concern, and prudent use of antibiotics is increasingly important
In this manner, other alternative treatments to relief the symptoms, prevent the recurrence and decrease the frequency of bacterial resistance are being investigated. However, there are no other alternative treatments worldwide accepted to treat UC.
Cranbrerry, the fruit of Vaccinium macrocarpon Aiton, contains proanthocyanidins (PACs) as the main components. Cranberry products may offer an alternative methodology to antibiotic therapy, and PACs are the main responsible for its activity.
There is one study already published comparing the use of a cranberry extract against Trimetoprim/Sulphametoxazol (TS) combination to prevent cystitis in premenopausal women9. According to results of this trial the efficacy of the TS treatment was slightly superior to cranberry extract (only 78% for antibiotic treatment vs 71% for cranberry treatment), to treat UC. Moreover, cranberry product was as safe as TS. However, the obtained data from this study shown that TS prophylactic uses induces a high percentage of bacterial TS-resistant, given that after one month of TS treatment the Ec isolates TS-resistant was 90%, in comparison with 28% en cranberry group.
Cranberry has been associated with the inhibition of bacterial adherence to uroepithelial cells. Cranberry extracts have been widely used to prevent recurrent cystitis although there is controversy about its efficacy on long-term treatments10-17. Data from different clinical studies are not always considered conclusive.
Since early 2000's it has been described that the effect of cranberry preparation was due to its proanthocyanidins (PAC) concentration: higher PAC contents leads to a better preventive effect18-19. PAC avoids the Ec adhesion to the inner urinary bladder wall and then UC could not occur because Ec cannot grow inside the urinary bladder and then bacteria flows out with the micturition. At the moment, only PACs form cranberry have shown this property.
Therefore, the content of the PACs in a cranberry product determines the effectiveness of a cranberry product in a dose-dependent manner. Different studies have shown that the inhibition of Ec adherence of cranberry PACs depends on the amount of PACs administered20-22. However, lack of specific data on differences among available cranberry extract used in the clinical trials, such as different methods of analysis for PACs standardization23, heterogeneity of the studies using either capsules or liquids or tablets with different unknown bioavailability and, perhaps lack of the most important data, their anti-adhesion activity, are making difficult to get well-based opinion on effectiveness and safety of a cranberry preparation. Hopefully that higher PACs concentration cause higher anti-adhesion activity. However this is not always showed due to lack of uniform PAC methods of analysis. There is not a PAC standardized method of analysis recognized by any Pharmacopoeia and it has been proven that PACs concentration is not always correlated with its anti-adhesion activity.
In fact, the last Cochrane revision highlights the need for quantified preparations using standardized methods to ensure the potency and enough content of PACs, since, probably the cause of the lack of efficacy observed in some preparations of cranberry juice is lower PACs amount administered24-25. Probably, the use cranberry extracts allows use products with a higher PACs concentration.
Cysticlean® is a medical device class II A in the European Union and it is recommended for the prevention and treatment of urinary tract infections. The objective of this pilot observational clinical trial has been to evaluate the efficacy and safety of Cysticlean®, a product with a cranberry extract with a high concentration of PAC (240 mg/capsule,) and an antiadhesión activity of 90%[Risco et al., manuscript in preparation] to treat UC instead of antibiotic.
MATERIALS AND METHODS
In an ambulatory practice, thirty consecutive patients, men and women aged 18 to 65 years, with UC were enrolled in this prospective observational, non-comparative study, performed in 3 months since recruitment started, between December 2014 and February 2015, at the Instituto Médico Tecnológico, (Barcelona, Spain). Informed written consent was obtained from all patients involved in the study. This study was carried out in accordance with the Helsinki Declaration (2000) of the World Medical Association and was performed by urologist in accordance with the principles of Good Clinical Practice.
UC was defined as a symptomatic cystitis, with pollakiuria, dysuria (painful voiding), urinary urgency (emergency for micturition), itching and gross hematuria (blood in the urine), and also positive urine-culture for Ec (Ec+). The inclusion criteria were that patients should have at least 2 of 5 signs/symptoms described above plus an Ec+. The exclusion criteria were currently treated with antibiotic or warfarin therapy, fever and general discomfort, and known allergy to cranberries. No other exclusion criteria were taken into account. Previous cystitis as well recurrent cystitis was not taking into account as inclusion/exclusion criteria. Not metabolic diseases were also taken into account as inclusion/exclusion criteria.
After enrollment, patients were instructed to take 1 capsule of Cysticlean® (240 mg PAC/capsule) every 12 h (morning and evening), until the second visit, two weeks later. Moreover, all patients were instructed to come back to immediately visit the doctor if signs/symptoms did not disappeared or patient felt Cysticlean® treatment was not good enough to relief symptoms of the UC. In these cases, Cysticlean® treatment was stopped and antibiotic treatment was prescribed according to the doctor's antibiotic ambulatory prescription for UC. Patients were followed up until UC signs and symptoms disappear.
Statistical analyses were performed using PASW Statistic 18 (SPSS) (2009, IBM Company, Chicago, Illinois). Standard descriptive statistics were used (mean ± standard deviation, max, min, and n) for continuous measurements. Univariate correlations (Pearson coefficient) between patient's characteristics (ages, sex and weight), number of signs and symptoms and the need for antibiotic therapy were calculated using a two-sided p-value. Comparative evaluation (t test) were done to compare male and female groups and patients group cured with Cysticlean® only versus and patients who needed to be cured with antibiotic. p-value of <0.05 was considered to be statistically significant.
Finally, an evaluation of the criteria to support this study design was included in the discussion.
RESULTS
All patients included in this study were ambulatory patients and they correspond to the first thirty patients (17 women and 13 men), who satisfied the inclusion/exclusion criteria, accepted to be enrolled and signed the written informed consent. All patients were recruited in less than 2 months. All included men had episodes of prostate hypertrophy but only two were under alfa-blocker treatment in the baseline.
Baseline characteristics of these patients showed no significant differences, between women and men, among evaluated parameters with the exception of a higher body weight in the case of men (Table 1). The whole group showed a normal distribution (Kolgomorov) and no abnormal values were found (Grubbs). At the beginning of the study all patients have at least 2 of 5 signs/symptoms of UC (36.6% with 2 signs/symptoms, 26.7% with 3 signs/symptoms, 26.7% with 4 signs/symptoms and 10% with 5 signs/symptoms) and a Ec+.
The treatment with Cysticlean® only achieved complete remission of signs and symptoms in 70% of patients (21 patients), 14 women and 7 men corresponding to 82.3% and 53.8% of women and men group, respectively (Table 2). According to these results, treatment of UC with Cysticlean® is more effective in women. These women continued the treatment with Cysticlean® to reduce potential recurrences.
The not responding patients, only 9 patients, were treated with established rescue medication because signs and symptoms persisted and Ec+ was still detected. Antibiotics used were septrin (trimetroprim/sulphametoxazole) (4), norfloxacin (2), ciprofloxacin (2) and phosmomycin (1). All antibiotics were effective in this study. Differences between women and men not cured with Cysticlean® alone were not evaluated because the number of patients was too low (3 and 6 respectively).
At the end of the study, all patients were cured, no signs/symptoms of UC were recorded and Ec was negative, including those who needed antibiotic treatment. The mean of the treatment duration was 22.8 days, slightly longer for women (24.6 days) than men (20.4 days.).
Differences between patients cured with Cysticlean® only (21 patients) and those who needed antibiotic (9 patients) were also evaluated. Statistically significant correlations were found between age and need for antibiotic therapy (p<0.05). It was found that those who needed antibiotic were significantly older (r=0.436, p=0.016), but number of signs/symptoms was not significantly higher in those patients treated with antibiotics (r=0.095, p=0.619). Moreover, neither sex nor weight could predict which patients will need antibiotic therapy (p >0.05).
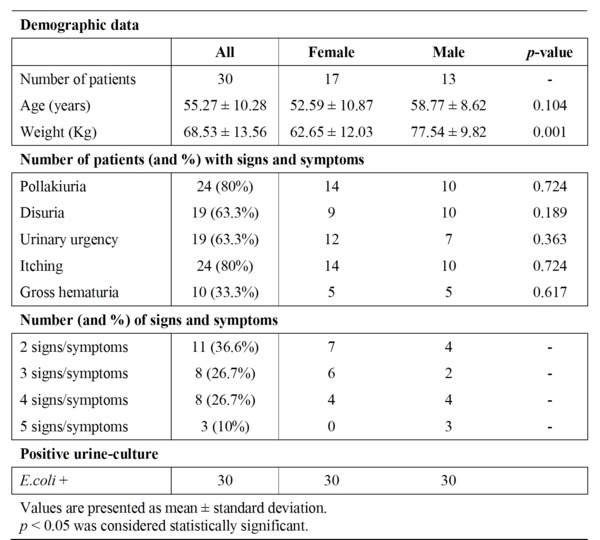
Table 1 Baseline characteristics of the patients.
Patients who needed antibiotic were going to visit the doctor before than those who did not (p <0.0001). However there were not differences between time duration of patients to be cured with Cysticlean® only (28.33 days) and patients who need antibiotic (25.56 days.) (p >0,05) (Table 2).
Men and women were analyzed separately in order to looking for any difference that could justify the incidence of antibiotic treatment need. Although the incidence of number of signs/symptoms between men and women was not statistically significant (p >0.05), men (6 of 13 men) had significantly (p<0.05) needed more antibiotic treatment than women (3 of 17 women). Moreover, there was also no evidence of a correlation between the number of signs/symptoms and needs of antibiotic therapy to cure the UC in the group of men (p = 0.883).
Additionally, it was possible to evaluate the patients perception related to their signs and symptoms and time to achieve the cure. They lasted to go to visit the doctor because they did not feel cure with the Cysticlean® treatment prescribed only (Figure 1). It is possible to see that almost all not cured patients went back to visit the doctor within 9 days after Cysticlean® treatment started. However 1 patient lasted up 22 days before he visited the doctor asking again for antibiotic treatment.
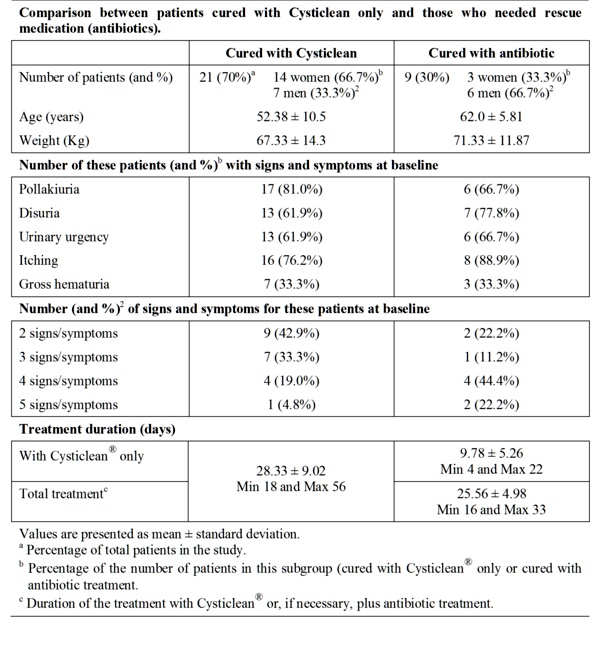
Table 2 Evolution of patients after treatment with Cysticlean©.
No side effects or adverse reactions were reported by any patients, treated with Cysticlean® or antibiotics, and/or doctors.
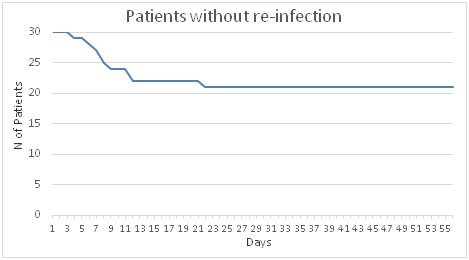
Figure 1.- Evolution of the number of patients without re-infection.
DISCUSSION
This observational study is one of the first done with a cranberry product (Cysticlean® capsules) with a high PACs concentration (240 mg/capsule), with an anti-adhesion activity up to 90% [unpublished data]. The obtained results show Cysticlean® as a safe and effective therapeutic option in the treatment of patients with UC.
There are many cranberry products commercialized world-wide as food supplements and very few as medical devices in the EU, but it is not easy to find published clinical studies with cranberry products who declare their PACs content as well as its anti-adhesion activity. There is only one study that has proven that three times its recommended clinical dose is three times more powerful reducing the Ec adhesion18. Even there is no studies published confirming that high anti-adhesion activity is more effective than low to cure UC, there are not too many studies published treating this infection with a cranberry product only.
In addition to the difficulty to compare the PACs concentration and its correlation with the anti-adhesion activity, it has not been possible yet to perform an accurate evaluation whether cranberry preparation can been used as alternative of antibiotics to treat and prevent UC.
This study has included women and men, 17 and 13, respectively, without any restriction apart from previous antibiotic treatment and known cranberry allergy. Subjects enrolled were all ambulatory patients. All patients were recruited in less than 2 months. The first 30 patients, who satisfy inclusion/exclusion criteria and who accepted to cranberry product as monotherapy, were enrolled in this study and were treated with Cysticlean® alone. Antibiotics can been used as a rescue medication when signs and symptoms of UC did not disappear with this treatment.
At baseline, all patients had a Ec+ and all patients ended the treatment without symptoms of and a negative Ec urine-culture. The treatment duration was not significantly different between patients treated with Cysticlean® alone and patients treated with Cysticlean® and antibiotics.
Only 30% of patients (3 women and 6 men) needed antibiotics to cure their UC. If we take into account the women group only, 82.35% of women enrolled did not need antibiotic to successfully cure, whereas 53.85% of men enrolled did not need antibiotic to successfully cure. Due to the small sample of men and lack of collected data of their prostate status it is not possible to conclude whether men with known history of prostate hypertrophy had a different behavior. 1 man under alfa-blocker treatment was successfully cured with Cysticlean® only whereas the other patient needed antibiotic.
All women who successfully respond to Cysticlean® treatment continued with this therapy during three more months, to help to reduce the re-infection within first 2 month after the first episode.
The comparative high number of male recruited in this study could be explained because it was done by urologists whereas women are mainly treated by general practitioners and/or gynaecologists.
Time among 1st and 2nd visit was longer than scheduled especially for those patients cured with Cysticlean®. It could be explained because when the study was done it was a Christmas season and patients who felt cured decided to go to visit the doctor later than scheduled. Perhaps patient's perception of symptoms/signs was not being felt as serious as to need to visit the doctor earlier. The level of perception (mild/moderate) is quite common among these patients. This could be applied also to those who need antibiotic (perhaps signs and symptoms were not perceived as severe enough). UC is not always perceived as a severe disease, especially among who had already suffered this pathology. According to the records obtained, no one patient took another drug/treatment.
As a "series of consecutive cases" design, this study has not a comparative group and then it is very difficult to conclude whether in a double blind randomized study results will be as good as those obtained in this one. However there are several criteria that could help to better consider this design, especially when not previous studies are available. First, goals and objectives are clearly defined, second, patients are quite representative taking into consideration age, weight and the disease treated, third, patients use to be visited in the same centre and by the same urologists, fourth, UC treatments are standardized, fifth, there were not missing data, sixth, methods to validate the treatments are already extensively known and did not change during the study and, seventh, no patients were lost in the follow up26.
Side effects/adverse reactions were neither described with Cysticlean® treatment. No complications were found in this observational study and patients' health was never at risk as it has been shown with the obtained results and safety profile described. Furthermore, the use of cranberry products, as Cysticlean®, to reduce antibiotic resistance would be an important therapeutic advantage, and preliminary data using well defined cranberry product to treat UC should be generated in order to be able to perform further more sophisticated studies to confirm this hypothesis.
CONCLUSSION
These preliminary data strongly suggest that well defined Cysticlean® which cranberry product with a high PAC concentration and a high anti-adhesion activity could be taken into account as an alternative to antibiotics for a 1st line treatment of UC. Further clinical studies to confirm whether Cysticlean® could be an alternative to antibiotic treatment for UC and this approach could contribute to reduce world-wide growing antibiotic resistance.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
ACKNOWLEDGEMENTS
The authors thanks to Professor J.F. Macias-Núñez for his help in the preparation of the manuscript
REFERENCES
1.- Grabe M, Bartoletti R, Bjerklund-Johansen TE, Çek HM, Pickard RS, Tenke P,Wagenlhener F, Bullt B: Guidelines on urological infections. European Association of Urology, 2014. Available at http://uroweb.org/wp-content/uploads/19-Urological-infections_LR.pdf. Accessed 5 May 2015.
2.- Mirone V, Franco M. Clinical aspects of antimicrobial prophylaxis for invasive urological procedures. J Chemother. 2014;26:S1-S13.
3.- Solorzano A, Jimenez-Pacheco A, de Dios Luna del Castillo J, Sampedro A, Martínez-Brocal A, Miranda-Casas C, Navarro-Marí JM, Gutiérrez-Fernández J: Evolution of the resistance to antibiotics of bacteria involved in urinary tract infections: a 7-year surveillance study. Am J Infect Control. 2014;42:1033-1038.
4.- Wangenlehner FM, Weidner W, Naber KG: An update on uncomplicated urinary tract infections in women. Curr Opin Urol. 2009;19:368-374.
5.- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD: Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6.- Grigoryan L, Trautner BW, Gupta K: Diagnosis and management of urinary tract infections in the outpatient setting. JAMA. 2014;312:1677-1684.
7.- Gupta K, Bhadelia N: Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin North Am. 2014;28:49-59.
8.- Gobernado M, Valdés L, Alós JI, García-Rey C, Dal-Ré R, García-de-Lomas, Spanish Surveillance Group for Urinary Pathogens: Antimicrobial susceptibility of clinical Escherichia coli isolates from uncomplicated cystitis in women over a 1-year period in Spain. Rev Esp Quimioterap. 2007;20:68-76.
9.- Beerepoot MAJ, ter Riet G, Nys S, van der Wal WM, de Borgie CAJ, de Reijke TM, Prins JM, Koeijers J, Verbon A, Stobberingh E, Geerlings SE: Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171:1270-1278.
10.- Salo J, Uhari M, Helminen M, Korppi M, Nieminen T, Pokka T, Kontiokari T: Cranberry juice for the prevention of recurrences of urinary tract infections in children: a randomized placebo-controlled trial. Clin Infect Dis. 2012;54:340-346.
11.- Dugoa J, Seely D, Perri D, Mills E, Koren G: Safety and efficacy of cranberry (Vaccinium macrocarpon) during pregnancy and lactation. Can J Clin Pharmacol. 2008;15:e80-e86.
12.- Wing DA, Rumney PJ, Preslicka CW, Chung JH: Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. J Urol. 2008;180:1367-1372.
13.- Garat Barredo JM: Tratamiento de las infecciones urinarias en pediatría con extracto de arándano rojo Americano. Acta Pediatr Esp. 2011;63:117-120.
14.- Ballester FS, Vidal VR, López Alcina E, Domenech Pérez C, Escudero Fontano E, Oltra Benavent AM, Montoliu García A, Sobrón Bustamante MA: Cysticlean a highly PAC standardized content in the prevention of recurrent urinary tract infections: an observational, prospective cohort study. BMC Urol. 2013;13:28.
15.- Collado A, Trassierra M, Monllor E, Navalón P, Tramoyeres A, Ordoño F, Osca J, Gómez A, Monzonís L, Dumont R: Estudio observacional del tratamiento con un extracto de arándano rojo Americano rico en proantocianidinas (PAC 118 mg) para el tratamiento de las infecciones urinarias de repetición. Urol Integr Invest. 2009;14:366-369.
16.- Bonet I, Batista E, Conejero J, Cortadellas L, Mandaña A, Peyrí E, Pigrau A, Urmeneta JM, Vargas C, Viladoms JM: Arándano rojo en el tratamiento de la cistitis urinaria. Urol Integr Invest. 2008;13:214-217.
17.- Vidlar A, Vostalova J, Ulrichova J, Student V, Stejska D, Reichenbach R, Vrbkova J, Ruzicka F, Simanek V: The effectiveness of dried cranberries (Vaccinium macrocarpon) in men with lower urinary tract symptoms. Br J Nutr. 2010;104:1181-1189.
18.- Howell AB, Botto H, Combescure C, Bland-Potard AB, Gausa L, Matsumoto T, Tenke P, Sotto A, Lavigne JP: Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidins content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94.
19.- Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE: Cranberry products inhibit adherence of P-fimbriated Escherichia coli to primary cultured bladder and vaginal epitelial cells. J Urol. 2007;177:2357-2360.
20.- Di Martino P, Agniel R, David K, Templer C, Gaillard JL, Denys P: Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: a double-blind randomized placebo-controlled cross-over trial. World J Urol. 2006;24:21-27.
21.- Lavigne JP, Bourg G, Combescure C, Botto H, Sotto A: In vitro and in vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect. 2008;14:350-355.
22.- Risco E, Miguélez C, Sánchez de Badajoz C, Rouseaud A: Effect of american cranberry (Cysticlean) on Escherichia coli adherence to bladder epithelial cells. In vitro and in vivo study. Arch Esp Urol. 2010;63:422-430.
23.- Kelm MA, Hammerstone JF, Schmitz HH: Identification and quantification of flavonols and proanthocyanidins in foods: How good are the datas?. Clin Develop Immunol. 2005;12:35-41.
24.- Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321.
25.- Jepson RG, Williams G, Craig JC: Cranberries for preventing urinary tract infections. Sao Paulo Med J. 2013; 31:363.
26.- Moses LE: The series of consecutive cases as a Device for Assessing Outcomes of interventions. Ed.: John C. Bailar III & Frederick Mosteller, NEJM Books, 1986. Chapter 6, Medical Uses of Statistics.
CORRESPONDENCE:
Ester Risco Rodríguez
Phytonexus S.L.
C/Salvadro Espriu, 27. 08253
Sant Salvador de Guardiola
Barcelona, Spain.
Mail: ester.risco @ phytonexus.com
Comment of the reviewer Dra. Ines Staneloni. Infectious Diseases Section of Internal Medicine Division, and Coordinator of Infection Prevention Committee. Hospital Italiano de Buenos Aires, Argentina.
The emergence of antimicrobial resistance is according to the World Health Organization a growing threat to global public health.
In Argentina, and specifically regarding urinary infections, we observed increased resistance to one of the most frequently used for this pathology antibiotics compromising its effectiveness.
Here we share some data about the evolution of quinolone resistance in strains of Escherichia coli in Argentina:
Comment of the reviewer Dr. Astrid Smud. nfectious Diseases Section of Internal Medicine Division, Hospital Italiano de Buenos Aires, Argentina.
We are going through a health problem worldwide due to increased antibiotic resistance. This is not limited to the hospital setting but crossed these barriers to be installed in community infections such as urinary tract infections, abdominal, skin and soft tissue among the most common. That is why we should be very cautious when using antibiotics to not continue working with this crisis.
As we are dealing with a positive urine culture paramount is to differentiate between asymptomatic bacteriuria and true urinary tract infection as asymptomatic bacteriuria (if we ignore urological prophylaxis) should not be exceptions such as renal immediate post-transplant and pregnancy, both indications increasingly discussed.
The use of alternative treatments to classic antibiotics for urinary tract infections are a very important point. This paper presents the limitations of being a, non-comparative observational study with a small number of patients, so it could not, without further study, recommending it yet as a first line of treatment. However, in patients with recurrent urinary tract infections by E. coli can be used as prophylaxis combined with dietary hygiene measures and the use of local estrogen in postmenopausal women to try to reduce recurrences and, with this, the use of antibiotics in ambulatory practice.
