Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
SMALL VESICLES, BIG VEHICLES: EXOSOMES.
Patricia Saiz-Lopez1, Raquel Alcaraz2, Enrique García-Toro1
1Servicio de Anatomía Patológica. 2Unidad de Investigación.
Hospital Universitario de Burgos.
Burgos. España
saizpa @ hubu.es
Rev Electron Biomed / Electron J Biomed 2016;3:41-48.
Comment of the reviewer David Ontoso Picon, MD. PhD. Molecular Biology. Memorial Sloan Kettering Cancer Center, Nueva York. USA.
Comment of the reviewer Prof. Carlos I. Lorda Diez, PhD. Departamento de Anatomía y Biología Celular, Facultad de Medicina, Universidad de Cantabria - IDIVAL. España.
SUMMARY:
Exosomes are small membranous vesicles released by different cell types. Since their discovery, they have evolved from being considered simple vehicles for the liberation of cellular wastes, to become one of the most promising fields in the area of biomedical research, and more specifically in oncology, since the different malignant tumors release exosomes to all biological fluids, being involved in various functions of the neoplastic process.
At present, it is possible to study these vesicles by minimally invasive techniques in patients, which approach us to obtain a more detailed diagnosis and prognosis, as well as to the discovery of new antitumoral therapies.
KEY WORDS: Cancer. Biomarker. Liquid biopsy
RESUMEN: PEQUEÑAS VESÍCULAS, GRANDES VEHÍCULOS: LOS EXOSOMAS
Los exosomas son pequeñas vesículas de membrana liberadas por diferentes tipos celulares. Desde su descubrimiento, han pasado de considerarse meros vehículos de liberación de deshechos celulares, a convertirse en uno de los campos más prometedores en el área de investigación biomédica, y más concretamente oncológica, ya que los diferentes tumores malignos liberan exosomas a los distintos fluidos biológicos, estando involucrados en numerosas funciones del proceso neoplásico.
Actualmente, se puede recurrir al estudio de estas vesículas mediante técnicas mínimamente invasivas para los pacientes, que nos aproximan a la obtención de un diagnóstico y pronóstico más acotado, así como al descubrimiento de nuevas terapias antitumorales.
PALABRAS CLAVE: Cáncer. Biomarcador. Biopsia líquida
INTRODUCTION
Exosomes are small membranous vesicles between 30-150 nm in diameter, released by exocytosis by different cell types, including tumor cells, and present in multiple biological fluids1. They were first described in 19832, and their biological function has been accepted for years as a form of cell debris release3. A decade later their mediating role has been confirmed in cell-cell communication4.
Remarkably, tumor cells have been shown to release a greater number of exosomes than the normal ones5. Matsumoto et al.6 describe the quantification of plasma exosomes as a prognostic biomarker of esophageal squamous cell carcinoma by determining that patients with this type of cancer had higher levels of exosomes than patients with non-malignant conditions.
The aim of this review is to demonstrate the clinical potential of exosomes in the diagnosis of cancer.
Origin, structure and composition of exosomes
In a eukaryotic cell, endocytic vesicles of the plasma membrane fuse to form early endosomes that mature to late endosomes5,7.
These structures are known as multivesicular bodies (MVBs) because they contain many vesicles called endosomal vesicles (intraluminal endosomal vesicles, (ILV)). When MVBs fuse with the plasma membrane, they release their ILV into the extracellular space in an exocytois process 8; It is at this time that these vesicles are called exosomes 9.
It has been suggested that heparan sulfate proteoglycans and synthetin control the formation of exosomes 10-11, whereas secretion is regulated by the Rab GTPase pathway 12-13. In addition, factors such as a decrease in the pH of the cellular microenvironment, favor the release of exosomes and their uptake into recipient cells 14
Exosomes have a simple structure, composed of a phospholipid bilayer, which, in comparison with the cell membrane, is richer in cholesterol and sphingomyelin 15. At first it was thought that the exosomes were homogeneous, however today it is known that their composition varies according to the origin and cellular state 16. For example, exosomes of a specific type of tumor will express specific markers, as in the case of melanin A in melanomas17.
Numerous proteins could be present on their surface , including CD63, CD81, CD82, CD53 and CD37, which are used as markers of exosomes 18, as well as EGF or TGF-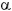 , which are relevant as EGFR ligands 19.
Their nucleus is aqueous, so they can compartmentalize and protect numerous molecules from being degraded, including proteins, lipids, messenger RNAs and microRNAs 20-21. Among the proteins are the annexins, GTPases, heat shock proteins and MVB synthesis proteins (Alix and TSG101).
Another important property of their structure is that it allows them to be distributed easily by the organism, and can even cross the blood-brain barrier. Therefore, exosomes are able to transport various biological components to very distant receptor cells that are able to absorb them, so that they take part in intercellular communication in a wide range of circumstances, from pathological to physiological. Taking advantage of these characteristics, they can be used as anti-tumor drug carriers. For example, it has been discovered that paclitaxel is 50 times more potent encapsulated in exosomes in the treatment of patients with lung cancer 22.
In this way, exosomes can be found in various biological fluids including serum, plasma, urine, amniotic fluid or breast milk. This fact allows introducing the term of liquid biopsy with the benefits of obtaining them from the patient by minimally invasive methods. Their isolation in the fluid and the methods for analyzing exosomes are fundamental to know them in depth, emphasizing in the current investigations the centrifugation systems and analysis of miRNAs, although there are novel lines of isolation in development 23-24.
, which are relevant as EGFR ligands 19.
Their nucleus is aqueous, so they can compartmentalize and protect numerous molecules from being degraded, including proteins, lipids, messenger RNAs and microRNAs 20-21. Among the proteins are the annexins, GTPases, heat shock proteins and MVB synthesis proteins (Alix and TSG101).
Another important property of their structure is that it allows them to be distributed easily by the organism, and can even cross the blood-brain barrier. Therefore, exosomes are able to transport various biological components to very distant receptor cells that are able to absorb them, so that they take part in intercellular communication in a wide range of circumstances, from pathological to physiological. Taking advantage of these characteristics, they can be used as anti-tumor drug carriers. For example, it has been discovered that paclitaxel is 50 times more potent encapsulated in exosomes in the treatment of patients with lung cancer 22.
In this way, exosomes can be found in various biological fluids including serum, plasma, urine, amniotic fluid or breast milk. This fact allows introducing the term of liquid biopsy with the benefits of obtaining them from the patient by minimally invasive methods. Their isolation in the fluid and the methods for analyzing exosomes are fundamental to know them in depth, emphasizing in the current investigations the centrifugation systems and analysis of miRNAs, although there are novel lines of isolation in development 23-24.
Function of exosomes in the development of cancer
In contrast to the initial studies on the release of exosomes as a mechanism to remove cell depletion molecules25, other studies proposed a functional communication between exosomes and cells. At present, they stand out for their role in carcinogenesis.
Once recipient cells receive the exosomes, the transported molecules are able to modify the cascades of cellular signaling, being able to trigger the initiation of the tumor process or metastasis, as well as being able to make them resistant to anti-tumor treatments27. This process is now considered to be non-random, but is dependent on membrane proteins26.
Melo et al.28 described that tumor-derived exosomes may promote tumor formation by regulating miRNA synthesis by the miRNAs transferred by the exosomes, affecting tumor initiation and progression.
In addition, tumor cells can promote angiogenesis, favoring tumor growth. Cui et al.29found that exosomes derived from lung cancer cells can selectively transport miRNA-210 to endothelial cells and promote the formation of new tumor blood vessels.
Another relevant finding is that the analysis of the long non-coding RNA content (lncRNA) in exosomes of samples from patients with prostate, gastric and laryngeal squamous cell carcinomas, has served to differentiate cancer cells from normal cells30, which opens the door to new methods of cancer detection.
Similarly, higher levels of CD63 + blood exosomes were detected in patients with melanoma, compared to healthy volunteers31.
It should be noted that the affinity for metastasizing to an organ has also been related to specific markers of the exosomes. Patterns of 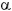 6
6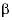 4 and
4 and 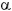 6
6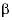 1 integrin expression in cancer cells have been linked to lung metastases, while other integrins were related to liver metastases 32. Therefore, this discovery could be used to predict the evolution of a tumor and generate new therapeutic targets against the metastatic process.
1 integrin expression in cancer cells have been linked to lung metastases, while other integrins were related to liver metastases 32. Therefore, this discovery could be used to predict the evolution of a tumor and generate new therapeutic targets against the metastatic process.
Therefore, it is important to be able to quantify, as well as molecularly characterize the exosomes in order to determine their function and clinical usefulness. For example, it has been suggested that glipican-1 could be used to detect early-stage pancreatic cancer 28 while miR-21 and miR-93 could be used to detect the early phase of hepatocellular carcinoma 33-34.
CONCLUSION:
The potential of tumor-derived exosomes to monitor the progression of malignant neoplasms, as well as in the early detection of malignancies, makes the exosomes the subject of promising research, not only by studying the load of exosomes in the different biological fluids, but by the characterization of their content in biomarkers, such as miRNAs.
REFERENCES
- 1.- Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86.
2.- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967-978.
3.- Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70(3-4):179-90.
4.- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161-1172.
5.- Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013;1:e201.
6.- Matsumoto Y, Kano M, Akutsu Y, Hanari N, Hoshino I, Murakami K, et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep. 2016;36(5):2535-2543.
7.- Raposo G1, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383.
8.- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923-935.
9.- Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125-134.
10.- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677-685.
11.- Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25(4):412-28.
12.- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19-30; sup pp 1-13.
13.- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513-525
14.- Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211-34222.
15.- Brouwers JF, Aalberts M, Jansen JW, van Niel G, Wauben MH, Stout TA, Helms JB, Stoorvogel W. Distinct lipid compositions of two types of human prostasomes. Proteomics. 2013;13(10-11):1660-1666
16.- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208-15.
17.- Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184(1):28-41.
18.- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397-422.
19.- Subramanian A, Gupta V, Sarkar S, Maity G, Banerjee S, Ghosh A, Harris L, Christenson LK, Hung W, Bansal A, Banerjee SK. Exosomes in carcinogenesis: molecular palkis carry signals for the regulation of cancer progression and metastasis. J Cell Commun Signal. 2016;10(3):241-249.
20.- Ratajczak J1, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487-1495.
21.- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654-659.
22.- Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655-664.
23.- Im H, Shao H, Weissleder R, Castro CM, Lee H. Nano-plasmonic exosome diagnostics. Expert Rev Mol Diagn. 2015;15(6):725-733
24.- Wu Z, Zeng Q, Cao K, Sun Y. Exosomes: small vesicles with big roles in hepatocellular carcinoma. Oncotarget. 2016;7(37):60687-60697.
25.- Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200(4):367-371.
26.- Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, Cao P. Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front Pharmacol. 2017;8:300
27.- Peng P, Yan Y, Keng S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep. 2011;25(3):749-762.
28.- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177-182.
29.- Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L, Ilie M, Hofman P, Nagase H, Mari B, Krüger A. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene. 2015;34(28):3640-3650
30.- Silva A, Bullock M, Calin G.. The Clinical Relevance of Long Non-Coding RNAs in Cancer. Cancers (Basel). 2015;7(4):2169-2182
31.- Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219.
32.- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329-335.
33.- Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, Shim SG, Paik YH. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015; 47:e184
34.- Wang H, Hou L, Li A, Duan Y, Gao H, Song X.. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894.
CORRESPONDENCE:
Dra. Patricia Saiz-Lopez
Servicio de Anatomía Patológica.
Hospital Universitario de Burgos.
Islas Baleares 3
09006, Burgos. España
Mail: saizpa @ hubu.es
CComment of the reviewer David Ontoso Picon, MD. PhD. Molecular Biology. Memorial Sloan Kettering Cancer Center, Nueva York. USA.
Los autores elaboran una acertada introducción sobre la complejidad de los exosomas como mensajeros intercelulares, tanto en situaciones fisiológicas, como tumorales. Para a continuación remarcar el tremendo potencial clínico que el empleo de exosomas supone tanto en diagnóstico, como en la elaboración de tratamientos farmacológicos punteros que superan las limitaciones tradicionales.
Comment of the reviewer Prof. Carlos I. Lorda Diez, PhD. Departamento de Anatomía y Biología Celular, Facultad de Medicina, Universidad de Cantabria - IDIVAL. España.
La revisión presentada por los autores incide en la importancia del análisis de exosomas en Biomedicina, ya que su contenido puede ofrecer mucha información en condiciones fisiopatológicas.
Actualmente, la comunidad científica presta cada vez una mayor atención a estos elementos, tanto para estudiar sus funciones como para entender las formas en las que se pueden utilizar en diagnósticos y tratamientos.
