Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
Original Paper
Olivar Clemente Castejon Sandoval PhD.1
Luzardo A Canache C, MD. PhD.2 Jesus Veroes, MD. PhD.3 Ana E Guerra, MD. PhD.3 Joyce Y Urdaneta Bcs. Msc.4
1Titular professor in Biology, Bsc, Msc. Coordinator of the laboratory of Electron Microscopy. Director of the Center of Research and Assistancel Teaching of the Aragua Nucleus (CIADANA). Faculty of Health Sciences, Carabobo University. Maracay-Aragua State,
2The Floresta Professional Center. The Floresta Maternity Annex. Maracay.
3Female Health Unity. Paula Saint Medical Group. Caracas-The Cafetal.
4Assistant of Research in the Aragua Nucleus (CIADANA).
Venezuela.
Email: olivar.ciadanauc @ gmail. com
3Female Health Unity. Paula Saint Medical Group. Caracas-The Cafetal.
4Assistant of Research in the Aragua Nucleus (CIADANA).
Venezuela.
Rev Electron Biomed / Electron J Biomed 2020;1:12-19.
Comentario de la revisora Dra. María Jesús Coma del Corral, MD. PhD Unidad de Investigación del Hospital Universitario de Burgos. España
Comentario del revisor Maria Teresa Tuñón Alvarez, MD. PhD. Prof Titular de Anatomía Patológica. Complejo Hospitalario de Navarra. Pamplona. SPAIN.
RESUMEN
Objetivos: El propósito de este estudio consiste en caracterizar la placenta, mediante el uso de microscopia de luz, infectada por Coronavirus en una paciente de 30 años de edad a las 33 semanas de embarazo quien a las 35 semanas obtuvo un feto muerto.
Métodos: La placenta fue obtenida con un hematoma de 7x8x2cm, infarto ocupando el 40% del volumen placentario, deposición de fibrinoide obliterando el 50% del espacio intervelloso, con corioamnionitis y calcificación distrófica. Esta fue comparada con una placenta control cuya paciente no tuvo antecedentes de alguna enfermedad.
Resultados: Espacio intervelloso muy reducido, la región estromal de las vellosidades está invadida por células mononucleares con vasos sanguíneos desorganizados o destruidos. Restos de vellosidades se observan en el espacio intervelloso. El sincitio en algunas observaciones está separado del estroma y sufre necrosis en otras vellosidades. Villitis y intervillositis se notan con frecuencia. Las vellosidades troncales sufren degeneración.
Conclusión: El tejido placentario se observa bajo una severa inflamación y destruido en algunas regiones, disminuyendo notablemente la población de vellosidades lo cual afecta el normal intercambio de gases y nutrientes provocando muerte fetal.
PALABRAS CLAVE: Fuerte Infección. Coronavirus. Degeneracion placentaria. Muerte Fetal.
ABSTRACT:
Objectives: The purpose of this study is to characterize the placenta using light microscopy, infected by Coronavirus in a patient of 30 years old to 33 weeks of pregnancy who to 35 weeks obtained a dead fetus.
Methods: The placenta was obtained with a 7x8x2cm Hematome, infarct occupying the 40% of the placental volume,fibrinoid deposition obliterating the 50% of the intervillous space, with chorioamnionitis and distrofic calcification. This was compared with a normal placenta whose placenta had not antecedents of any illness.
Results: The intervillous space is reduced. Stromal region of villi is invaded by mononuclear cells with blood vessels disorganized or destroyed. Debris of villi is observed in the intervillous space. The syncytio in any observations is separating of the stroma and suffers nechrosis in other villi. Villitis and intervillositis are noted with frecuency. The stem villi are suffering degeneration.
Conclusion: The placental tissue is observed under severe inflammation and destroyed in any regions reducing so notably the population of placental villi which affect the interchange normal of gases and nutrients provoquing fetal death.
KEY WORDS: Strong Infection. Coronavirus. Placental Degeneration. Fetal Death
INTRODUCTION:
Little is known about maternal and neonatal outcomes of infections by coronavirus in the first and second trimesters of pregnancy. High levels of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) invading the intervillous space within placenta are contributing with the placental inflammation that ultimately resulted in early onset of preeclampsia and worsening maternal condition1.
Transmission electron microscopic analysis of placental tissue showed particles of 75-100nm in diameter consistent with the size and appearance of SARS-CoV22. Viral infections may predispose pregnant woman to higher rate of pregnancy complications, including preterm birth, miscarriage and stillbirth. Pregnant woman are more susceptible to SARS-CoV2 infections in adverse outcomes when is associated to obesity, diabetes or hypertension. Coronavirus may cluster on the syncitio and their colonization provoques local inflammation and damage. The human angiotensing-converting enzyme II (ACE2), the receptor, and the human transmembrane protease serine 2(TMPRSS2) facilitates viral invasion3.
Infected the placental villi can to occur vertical transmission. In third trimester following maternal SARS-CoV2 infection the histopathologic results of placenta showed maternal vascular malperfusion, fetal vascular malperfusion and evidence of inflammation as villitis, intervillositis and chorioamnionitis4. Decidual arteriopathy, villous agglutination, avascular villi, delayed villous maturation, chorangiosis, atherosis, fibrinoid necrosis, villous edema, intervillous thrombus and increased perivillous fibrin were found in 16 patients with SARS-CoV25.
Placental histological analysis reveals thrombosis, infarcts, and vascular wall remodeling on placental vessels in placentas from woman with Covid-19.Some infants born to mothers with confirmed Covid-19 have displayed problems including fetal distress,lethargn, vomiting, fever, respiratory distress, thrombocytopenia and death. ACE2 has seen proposed to promote maternal vasodilation, and is present in cyto and syncytiotrophoblast layer. SARS-CoV2 induces endothelitis. These observations are indicating fetal and maternal malperfusion. A higher number of Hofbauer cells is observed in placentas from woman with severe Covid-196.
The affinity of SARS-CoV2 spike protein to the human ACE2 is 10-20 fold higher than that of the SARS-CoV spike protein explaining why this transmission is more efficient and aggressive that previous coronavirus7. In a systematic review on 324 pregnancies with Covid-19 it was reported 9 cases of maternal deaths, 4 cases of spontaneous abortion, 4 cases of intrauterine fetal deaths and 3 cases of neonathal death8.
Maternal hypoxia secondary to severe Covid-19 lung infection may initiate uterine underperfusion and subsecuent hypoxic-ischemic injury to the placenta provoquing avascular villi, delayed villous maturation, chorangiosis, villous stromal karyorrhexis, villous oedema, subchorionis necrosis, vasculitis, intervillositis, chorioamnionitis and infarction9.
These viral particles were identified by first time in syncytio, endothelial cells, fibroblasts, maternal macrophagues, Hofbauer cells and fetal intravascular mononuclear cells in placentas with inflammatory infiltrate consisting of neutrophils and monocyte-macrophagues using immunohistochemic, electron microscopy and molecular analysis of placenta10.
MATERIAL and METHODS:
Two groups of placental villi were taken of placenta study and placenta normal. The group study proceeds from hospitalary institution whose placenta was obtained to the 35 weeks. The patient of 30 years old was infected during the 33 weeks of pregnancy manifesting symptoms of infection by Coronavirus. This was confirmed by nucleic acids detection by RT-PCR in the Hygiene National Institute-Caracas. The placenta showed a 7x8x2cm hematome, infarct occupying 40% of placental volume, extending from maternal face until chorionic plate, with extense deposition of intervillous fibrinoid obliterating 50% of the intervillous space, chorioamnionitis and distrophic calcification.
The serology of patient with placenta study was negative for hepatitis B, C, cytomegalovirus, Epstein Bar virus, rubella virus and toxoplasmosis. Without other metabolic disease, genetic, parasitary or with bad-formations and being seronegative to the six weeks of birth. The newborn was born death and the placental weight was of 465 gr.The patient had knowledge of informed consent and approval by the ethical committee of the hospitalary institution for the realization of this investigation according to the Helsinki Declaration.
The placenta normal was obtained to 38 weeks of patient with increase of weight of 10Kgr without antecedent of disease. Of each placenta were taken five small specimens of the maternal surface selected to the random from the region central parabasal in the vertical plane. Three slides by specimen were prepared for light microscopy and 30 histologycal slides in total were stained with H&E for their observation. Cross sections of placental villi stained with H&E were compared with similar regions in both groups of placentas
RESULTS:
Extense zones of placental villi have very reduced the intervillous space as regions of pre-infarcts. Stromal region of stem villi contains an accented invasion of mononuclear cells and much of these villi are suffering of chorangiosis although there are numerous zones of remarkable avascularity.
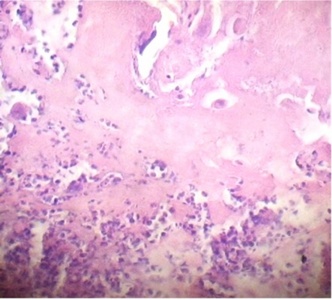
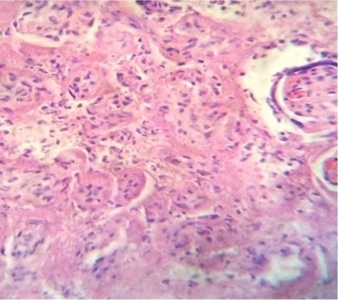
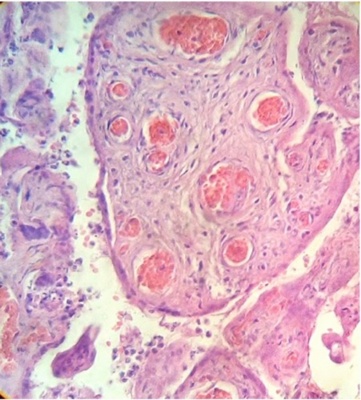
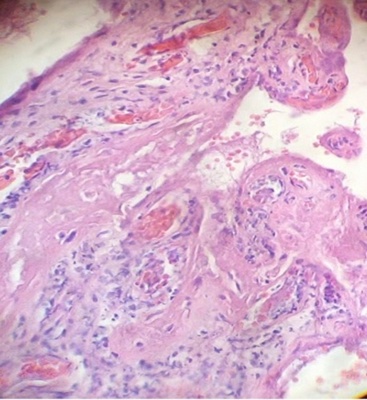
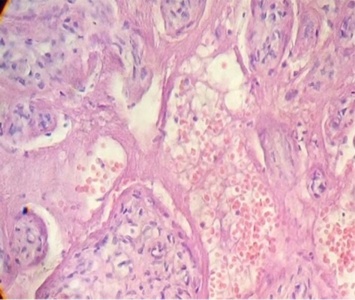
Regions of infarcts in degeneration with frecuency are seen. The majority of the vessels in the villi are disorganized or destroyed. Debris of placental villi is noted. The infiltration of mononuclear cells in the decidual region has provoqued the lysis of this zone in some regions not observing the plasma membrane of decidual cells and necrotic regions are evident (Fig.1). The placenta attacked by these virus shows villi with stromal region separating of the syncytio (Fig.2). The strong infection can be seen provoking villitis and intervillositis (Fig.3). Debris of placental villi are observed as plasma membrane, endothelium surrounding red blood cells and others fragments of villi (Fig.4).
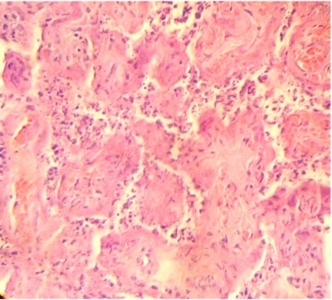
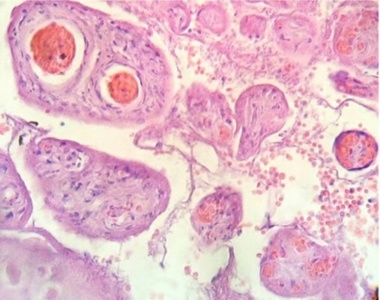
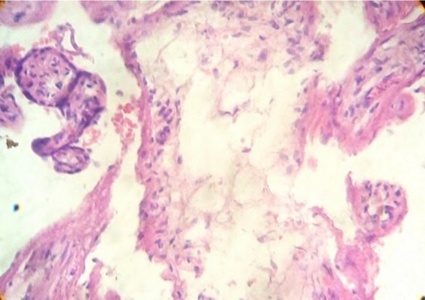
Part of a fragmented syncytio is separating of stromal region (Fig.5). Stem villi with ramifications in degeneration, deposition of fibrinoid and an intense infiltration of mononuclear cells are attracted by the tissue damage (Fig.6). These villi suffer of syncytial nechrosis and stromal region is separating of the syncytio (Fig.7). Ghost villi which presented stromal region severaly damaged and syncytio destroyed were noted as cellular debris in the intervillous space (Fig.8).
DISCUSSION:
Maternal infection by Coronavirus have more risk of fetal death that of a normal pregnancy, with poor uteroplacental blood flow, leading to numerous or large and acutely acquired placental infarcts that occur in mothers with pregnancy induced hypertension. The placenta gives protection against certain maternal infections but does not hinder passage of a number of viruses11.
This hypoxic condition produces chorangiosis associated to neutrophils, lymphocytes and plasma cells in the placental villi. The altered state of the endothelium in the decidua and chorionic villi of these placentas has revealed thrombosis and infarcts. Accelerate villous maturation, increased syncytial knots, villitis, intervillositis, vessel obliteration, avascular villi and increased number of Hofbauer cells has been the impact of Covid-19 on the placental structure6. Endothelitis provoked by this infection separates the syncytio of the stromal region which impede the normal villous development provoquing placental instability.
Placentas from pregnant woman with Covid-19 present inflammatory lesions including chronic histiocytes, intervillositis, villitis, funisitis, chorioamnionitis, chronic lymphocytic villitis, deciduitis, vasculitis, focal thrombosis, increased fibrin deposition, trophoblastic necrosis, inflammatory cells in the intervillous space and necrosis of villi11.
Coronavirus make use of diverse proteases for cellular entry, endosomal/lysosomal proteases, which may lead to destructive effect causing massive release of pro-inflammatory cytoquines and chemoquines resulting in inflammation, syncytial nechrosis, stromal edema and accumulation of mononuclear cells12.
During pregnancy, congenital viral infections can affect the health status of the fetus resulting in fetal death13.Nevertheless these virus have not been studied sufficiently in relation to the maternal-fetal interface .In a case of stillborn intrauterine transmission of SARS-CoV2 the placental analysis showed: accelerate villous maturation, increase of syncytial knots, decidual arteriopathy, fibrinoid necrosis, arterial thrombosis in decidual infarct, diffuse perivillous fibrin deposition, intervillositis, intervillous thrombi, increase of Hofbauer cells and histiocyte infiltrate14.
In our case an increase of Hofbauer cells was not noted in the observations since these were disappeared indicating that as macrophagues their activity was annulled by the viral load. The findings mentioned above also are observed in placentas of woman with hypertensive disorders. By other hand,Covid-19 in pregnant woman was associated with higher rates of mortality, being the maternal mortality of 1,3%15. In other different case of live birth in third trimester showed subchorionic deposition of fibrin, ischemic area, focal hemorrhages, delayed maturation, capillary congestion, microchorangiosis, villous agglutination, and intervillous hemorrhages evidencing inflammatory status16.Our recently published case showed deciduitis, stem degenerated villi, hypertrophied cytotrophoblasts cells originating multinucleated giant cells, destroyed villi in the intervillous space with distrophic calcification, infarction with numerous syncytial nodules and mature intermediate villi with numerous proportion of nucleus in the syncytio17.
In reality the histomorphological findings that can be observed during Covid-19 could be classified as signs of maternal malperfusion: infarction,fibrin deposition, decidual vasculopathy, intervillous fibrin, intervillous thrombosis and signs of fetal malperfusion: thrombosis fetal, avascular villi, karyorrhexis, delayed villous maturation and chorangiosis. Besides inflammatory changes as chorioamnionitis, chronic villitis, chronic deciduitis, subchorionitis, choriovasculitis and fetal vasculitis18. So, maternal vascular malperfusion lesions in the placenta are associated with stillbirth, preterm delivery and placental abruption in patients with Covid-1919.
There is evidence that this illness cause a severe systemic inflammatory response and may result in a hypercoagulable state with widespread microthrombi, thromboembolism and microangiopathic disease in almost every organ of our body20. This inflammatory change is similar to villitis of unknown etiology and could to answer the question whether infection with SARS-CoV2 increase the incidence of vascular pathology in the placenta21.
Mostly the infection has been studied during 3rd trimester. Data on 1st and 2nd trimester remain very limited. About a case in 2nd trimester this also showed maternal malperfusion, increased perivillous fibrin, infarctions, SARS-CoV2 in both trophoblasts, vascular hemolysis in terminal and intermediate villi, hemorrhage and lymphocytic-monocytic infiltration in decidua resulting in premature at 26th week who died 1 day after delivey22.
Gao L et al besides of these placental changes here mentioned have found distal villous hypoplasia, insufficient vessel remodeling and microscopic accrete in pathological examination of placentas of pregnant women with Covid-1923.
A possible explanation for intrauterine SARS-CoV2 infection is through placental barrier damage caused by severe maternal hypoxemia in women with Covid-1924 as observed in Fig.5.Five consecutive cases of fetal death occurred at 21-38 weeks of gestation with Covid-19 in women infected had acute chorioamnionitis, massive deposition of fibrin, intervillitis, villitis, intense neutrophil and lymphocyte infiltration with severe placental inflammatory reaction, deciduitis and ischemia25. Findings described in villitis of unknown etiology, related to hematogenous infection in placentitis and other viral infections21.
With a placenta associate to hematome great, 40% of placental volume infarcted and 50% of severe obliterating of the intervillous space our case presents a reduced proportion of placental villi leading to reduced maternal-fetal interface, to fetal hypoxia and fetal death as described by van Oppenraaij RHF et al provoqued by the reduced villous vascularization that affect the interchange of gases and nutrients26.
REFERENCES
- 1. Hosier H, Farhadian SF, Morotti RA, Desmukh U, Lu CA et al. SARS-COV2 infection of the placenta. J CLIN INVEST 2020; 130(9):4947-4953.
2. Goldsmith CS, Tamin A. Transmission electron microscopic imagen of an isolate from the first U.S. case of Covid-19, formerly known as 2019-nCoV.https://phil.cdc.gov/Details.aspx-?pid=23336.Accesed July2,2020.
3. Naomi KN, Ritter A, Louwen F, Yuan J. A messaje from the human placenta: Structural and Immunomodulatory defense against SARS-COV2.Cells 2020;9(8):1777.
4. Sharps MC, Hayes DJL, Lee S, Zou Z, Brady Ch A et al. A structured review of placental morphology and histopathological lesions associated with SARS-COV2 infection. Placenta 2020; 101:13-29.
5. Shanes ED, Mithal LB, Otero S ,Azad HA, Miller ES et al. Placental pathology in Covid-19.Am J Clinical Pathol 2020; 154(1):23-32.
6. Flores PA, Miranda J, Vega TS, Valdespino VY, Helguera RC et al. Molecular Insights into the trombotic and microvascular injury in placental endothelium of women with mild or severe covid-19. Cells 2021; 10(2):364
7. Elrashdy F, Elrashdy MR, Uversky VN. Why Covid-19 Transmission is more efficient and aggressive that viral transmission in previous coronavirus epidemics? Biomolecules 2020; 10(9); 1312.
8. Juan J, Gil MM, Rong Z, Zhang Y, Yang H et al. Effect of Coronavirus disease 2019 (Covid-19) on maternal, perinatal and neonatal outcome: Systematic review. Ultrasound Obstet Gynecol 2020; 56:15-27.
9. Ping WY, Yee KT, Ching TG. The effects of covid-19 on placenta and pregnancy: What do we know so far? Diagnostic (Basel )2021; 11(1):94.
10. Facchetti F, Bugatti M, Drera E, Tripodo C, Sartori E et al. SARS-COV2 vertical transmission with adverse effects on newborn revealed through integrated immunohistochemical, electron microscopy and molecular analysis of placenta. EBio Medicine 2020; 59:102951.
11. Schwartz DA, Morotti D. Placental pathology of Covid-19 with and without fetal and neonatal infection: Trophoblast nechrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV2.Viruses 2020; 12(11):1308.
12. Korner RW, Majjouti M, Alejandre AMA, Majabir E. Of mice and men: The Coronavirus MHV and mouse models as translational approach to understand SARS-CoV2.Viruses 2020; 12(8):880.
13. Leon JM, Martinez CM, Gonzalez GLD, Helguera RAC, Zaga CV et al. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog Dis 2017; 75(7):ftx093.
14. Stonoga ETS, de Almeida Lanzoni L, Zadoroshei RP, de Oliveira ALP, Chiste JA et al. Intrauterine transmission of SARS-COV2. Emerg Infect Dis 2021; 27(2):638-641.
15.Karimi L, Makvandi S, Vahedian AA, Sathyapalan T, Sahebkar A. Effect of Covid-19 on mortality of pregnant and postpartum women: A systematic review and meta- analysis. J Pregnancy 2021; Article ID 8870129:1-33.
16. Ferraiolo A, Barra F, Kratochwila Ch, Paudice M, Gaetano VV et al. Report of positive placental swabs for SARS-COV2 in an asymptomatic pregnant woman with covid-19. Medicina(Kaunas) 2020;56(6):306.
17.Castejon SOC, Canache CLA, Lara A, Veroes J. Infection by Coronavirus in the placental villi. Rev Electron Biomed/Electron J Biomed 2019;3:42-49.
18. Menter T, Diana MK,Jiang S, Chen H, Monod C et al. Placental pathology findings during and after SARS-COV2 infection: Features of villitis and malperfusion. Pathobiology 2020; Sept 18:1-9.
19. Patberg ET, Adams T, Rekawek P, Vahahian SA, Akerman M et al. Coronavirus diseases 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol 2021; 224(4):382.e1-382e18.
20. Connors JM, Levy JH. Covid-19 and its implications for thrombosis and anticoagulation. Blood 2020; 135:2033-2040.
21. Lewis SH and Perrin E. Pathology of the placenta. 2nd Edition. New York: Churchill Livingstone, Chap 13, Infectious disorders of the placenta by Hyde SR and Altshuler G, pp:330-331.
22. Sukhikh G, Petrova U, Prikhodko A, Starodubtseva N, Chingin K et al. Vertical transmission of SARS-COV2 in second trimester associated with severe neonatal pathology. Viruses 2021; 13(3):447.
23. Gao L,Ren J, Xu L, Ke Xiaokang, Xiong L et al. Placental pathology of the third trimester pregnant woman from covid-19. Diagn Pathol 2021; 16:8.
24. Wang C, Zhou YH, Yang HX, Poon LC. Intrauterine vertical transmission of SARS-COV2: what we know so far. Ultrasound Obstet Gynecol 2020; 56(6):724-725.
25. Richtman R, Torloni MR, Oyamada OAR, Levi JE, Tovara MC et al. Fetal deaths in pregnancies with SARS-COV2 infection in Brazil: A case series. Case Rep womens Health 2020; Jul 27:e00243.
26. van Oppenraaij RHF, Nik H, Heathcote L, McPortland JL, Turner MA, et al. Compromised chorionic villous vascularization in idiopathic second trimester fetal loss. Early Human Develop 2010; 86:469-472.
CORRESPONDENCE:
Prof. Olivar Clemente Castejon Sandoval
Coordinator of the Laboratory of Electron Microscopy.
Director of the Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA).
Faculty of Health Sciences, Carabobo University.
Maracay, Aragua State.
Venezuela.
Email: olivar. ciadanauc @ gmail. com