Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
Letters to the Editor / Cartas al Editor
HISTOLOGICAL ASSESSMENT OF EFFECTS OF ALCOHOLIC EXTRACT OF BENISEED ON REPRODUCTIVE ORGANS OF MALE SPRAGUE-DAWLEY RATS
Ebenezer Ashamu1, Oluyemi Kayode Alaba2, Ukwenya Victor O3, Omotuyi I.O4, F.I.O. Duru6, Ojo G.B3, Victor O. Makanjuola5, Okanlawon A. O6, Norohna CC6
1Department of Anatomy. School of Basic Medical Sciences. Ladoke Akintola University. Ogbomoso. Oyo State. Nigeria
2Department of Biology/Biotechnology, William Paterson University. Wayne. NJ. USA
3Department of Anatomy. Faculty of Basic Medical Sciences. College of Health Sciences. Bowen University. Iwo. Osun State. 4Department of Biochemistry, Adekunle Ajasin University. Akungba Akoko. Ondo State. 5Department of Anatomy. Faculty of Basic Medical Sciences. College of Medical Sciences. Bingham University. Nassarawa.
6 Department of Anatomy. College of Medicine. University of Lagos. Idi-Araba, Lagos State.
Nigeria.
kayodedanatomist @ yahoo.com
Rev Electron Biomed / Electron J Biomed 2009;3:73-76.
To the Editor:
It has been established that antioxidants such as Vitamins C and E are active enhancers of gonadal functions1-3. This is due to the fact that they scavenge free radicals (oxidants) in tissues and blood vessels neutralizing their damaging effects. Antioxidants increase gonadal functions by enhancing the hormonal secretions necessary for the proliferation of spermatogenic cells3
Beniseed oil has been implicated to regulate blood pressure to normal in adult hypertensive patients. Upon withdrawal, the blood pressures were elevated4. This might be due to the fact that it contains antioxidants such as sesamin (0.34 to 1.13%), sesamolin (0.13 to 0.58%) and sesamol. Sudesh & Vicki5 reasoned that antioxidants may achieve antihypertensive effects through one of three ways: reducing aldehyde conjugate/AGE formation and oxidative stress, normalizing calcium channels and peripheral vascular resistance, or by improving insulin resistance and endothelial function. In the presence of oxidants, constriction of the blood vessels and subsequent hypertension may result. In the same study, antioxidants (Vitamin C & E, b- carotene and reduced glutathione) were increased. These types of effects are pro-fertility in nature. We earlier argued that Beniseed enhances fertility by production of substances necessary for the proper function of6. Chang et al7stated that schisandrin B-sesamin mixture administered to male Sprague-Dawley rats exerted a hepatoprotective effects in CC4-induced oxidative stress. This plant seed was able to do this due to its antioxidant potency as earlier evaluated by Oluyemi et al8
Among all reactive oxygen species, hydrogen peroxide is the most toxic to testicular functions.9. Treatment with superoxide dismutase and catalase reduced the degree of testicular damage in experimental acute torsion by scavenging the superoxide radicals and converting them into hydrogen superoxide and water and the catalase then converts the hydrogen peroxide produced into water and oxygen10. These reactive oxygen species are regularly formed during the process of normal respiration. However, the production is kept at physiologically low levels by intracellular free radical scavengers11.
The seed contain 7-11% palmitic acid, 2-6% stearic acid, 39-56% Linoleic acid, 32-54% Oleic and other fatty acids in lesser than 1%. The plant seeds also contain two lignans which are oil-soluble: sesamin and sesamolin as well as lignan glucosides such as sesaminol di-glucoside and tri-glucosides, and pinoresinol mono-, di- and triglucosides12.
The present study aim at evaluating the effects of alcoholic extract of Beniseed seed on the architecture of selected reproductive organs.
Twenty (20) adult male rats of Sprague-Dawley strain obtained from the faculty of Basic medical sciences' animal house, Ladoke Akintola University of Technology, Ogbomoso, were used in this study. They weigh between 150.4g to 200.5g with mean weight of 200g. They were kept in iron cages at controlled room temperature of about 30 0C and 12:12 photo-periodicity. They were fed on rat pellet feed obtained from Ladokun feeds, Ibadan and water made available ad libitum. They were allowed to acclimatize for two weeks before the experiments.
Beniseed was obtained at a local market in Ogbomosho, Oyo State, Nigeria authenticated by the Botany Department, University Lagos. The seeds were peeled and sundried. They were finely powdered. The powdered form was allowed to stand in 70% alcohol for 24 hours and then filtered. The filtrate was concentrated to a semi-solid form using rotary evaporator. The Lethal Dose (LD50) estimation of beniseed was done at Pharmacognosy Department, College of Medicine University of Lagos and was found to be 5000mg/kg. A stock solution of the extract was prepared at a concentration of 300mg/ml. the solution was kept in the refrigerator to prevent fermentation. A known volume was dispensed daily for administration using an oropharyngeal canula.
The rats were divided into four (4) groups of 5 rats each. The groups were designated as control (C) and Treatments (T1, T2 ,T3)
Each of the rats in the treatment groups (T1, T2, T3) received a single dosage of 3000mg/kg body weight of extract solution, orally, daily for 8 weeks. T3 also received 2.5mg of Vitamin C (Tuyil Pharmaceutical Company Ilorin, Kwara State) in addition to beniseed extract. The animals were weighed once every week. After the initial period of 8 weeks, rats in T2 were withdrawn from beniseed extract for a period of 4 weeks. The animals were sacrificed by cervical decapitation as follows: C, T1 and T3 at the end of 8th week and T2 at the end of 12th week.
The testes, epididymides and seminar vesicles were removed by midline laparatomy. The epididymides were dissected free of the testes while the testes, epididymides and seminal vesicles were weighed in electrical weighing balance before fixing in 10% formol saline. The organs were histologically processed according to a routine method used and described by Oluyemi et al.8.
The results are presented in Figures 1 to 3
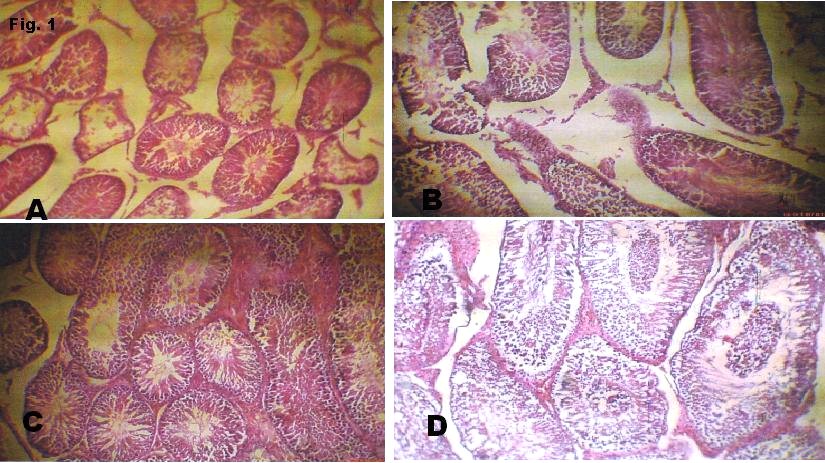
Figure 1
(b) Sections through testis of group T1 show elongated seminiferous tubules in longitudinal and cross sections. They possess stratified epithelia consisting of the full complement of cells of the spermatogenic series. The spermatogonia population increased compared to the control. There are numerous sertoli and leydig cells. Their basement membrane is of normal thickness. The stroma is normal.
(c) Sections through testis of T2 show regular uniformly spaced seminiferous tubules in longitudinal and cross sections. They possess a stratified epithelium consisting of the full complement of cells of the spermatogenic series. They show proper maturation from spermatogonia to the spermatids. The spermatogonia are limited to 1 layer while there are more spermatids. The sertoli cells are few. Their basement membrane is of normal thickness. The stroma thickened and contains numerous leydig cells.
(d) Sections through testis of T3 show dense thickened tunica albuginea containing dilated blood filled vessels. There are regularly shaped seminiferous tubules in longitudinal and cross section. Organized arrangement of cells of the spermatogenic series. The spermatogonia are shed into the lumina of tubules. Their basement membrane is of normal thickness. The stroma is thick with congested blood vessel and many leydig cells.
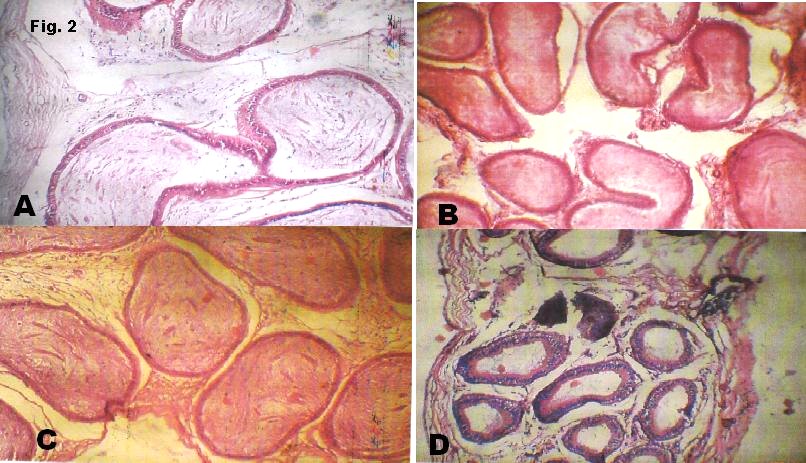
Figure 2
(b) Sections through the epididymis of the T1 show round tubes in cross sections lined by stratified epithelium with abundant stereocilia projection from their surface. These are surrounded by a thick muscle layer. Within the lumina are dense long eosinophilic processes of sperm tail.
(c) Sections through the epididymis of the T2 show round tubes in cross sections lined by pseudostratified columnar epithelium with stereocilia projection from their surface. These are surrounded by a thick muscle layer. Within the lumina are abundant long eosinophilic processes of sperm tail seen clumping together.
(d) Sections through the epididymis of the T3 show round tubes in cross sections lined by pseudostratified columnar epithelium with stereocilia projection from their surface. These are surrounded by single layered muscle cells. The lumen are virtually empty of sperm cells.
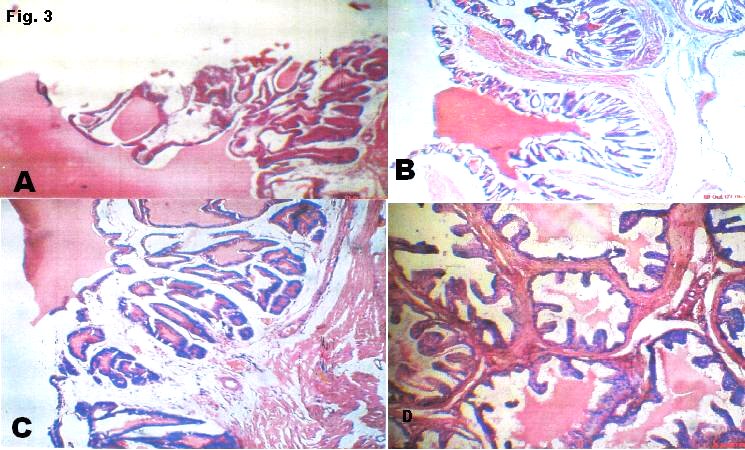
Figure 3
(b) Sections through the seminal vesicle of the T1 show glands glandular hyperplasia with more pronounced honeycomb arrangement. They are lined by tall columnar epithelium. The eosinophilic secretions within the lumen are reduced.
(c) Sections through the seminal vesicle of the T2 show glands arranged in a honeycomb fashion. They are lined by tall columnar epithelium. There is increased intraglandular secretion in addition to eosinophilic material within large lumen.
(d) Sections through the seminal vesicle of the T3 show glands glandular hyperplasia with labyrinthine projections. They are lined by stratified epithelium. There is increased secretion of eosinophilic material into a large lumen.
The photomicrographs of the testis of the experimental animals have proved that alcoholic extract of Beniseed seed has a profertility effects enhancing the production of spermatozoa and buffering the integrity of cells of spermatogenic series as well of the testicular cyto-architecture. These findings are in agreement with the findings of shittu et al13. In the presence of steroid-containing substances, spermatogenesis tends to become enhanced leading to the maturation and proliferation of spermatogenic cells. As can be seen in Fig. 1c and 1d, there were massive accumulations of spermatozoa in the lumen of the seminiferous tubules. This is an indication that beniseed extract is a potent fertility-enhancing plant. Earlier, we reported a dose-dependent increase in sperm counts in wistar rats6. There were observed increase in the thickness of seminiferous epithelia: a strong fact pointing to the proliferative effects of the plant. Beniseed oil induces developmental abnormalities in the male reproductive system of juvenile rats but these were restored at puberty14 as a result of distruption in the steroidogenesis, which is kept at base during childhood but shoots up at puberty.
The integrity of the epididymal cytoarchitecture were preserved in our experiment (Fig. 2a-2d) suggesting that Beniseed does not exert any adverse effects on the epididymis and the developing luminal spermatozoa as the latter further develop to acquire motility. This agrees with the work of Shittu et al.13.
Fig. 3a-3d show the effects of the plant seed on the secretory functions of the seminal vesicle. Seminal vesicles are responsible for the secretion of proteins, enzymes, fructose, mucus, vitamin C, flavins, phosphorylcholine and prostaglandins found in semen. This fructose serves as nutrition for the spermatozoa. Congenital absence of seminal vesicle is usually associated with absence of vas deference15. The extract shows an increased secretion into the canaliculi of the seminal vesicular network. This further proves that Beniseed seeds are potent antioxidant and hence a good profertility compound.
In experiment 3, the vitamin C was administered as adjuvant to increase the anti-oxidative function of Beniseed extract. In this class of experiment it was discovered that the presence of vitamin C does not alter significantly the antioxidant properties. In the overrall, the results show a progressive increase in the integrity of cytoarchitectures of the reproductive organs of the experimental animals.
These results thus confirm the traditional comsumption of Beniseed for the purpose of fertility treatment especially in males.
REFERENCES
1.- Ishihara M, Itoh M, Miyamoto K, Suna S, Takeuchi Y, Takenaka I, Jitsunari F. Spermatogenic disturbance induced by di-(2-ethylhexyl) phthalate is significantly prevented by treatment with antioxidant vitamins in the rat. Int J Androl. 2000;23:85-94.
2.- Marin-Guzman J, Mahan DC, Pate JL.. Effect of dietary selenium and vitamin E on spermatogenic development in boars. J. Anim. Sci. 2000 78:1537-1543
3.- Saalu L C, Oluyemi KA, Omotuyi IO. a-Tocopherol (vitamin E) attenuates the testicular toxicity associated with experimental cryptorchidism in rats. African J Biotechnol. 2007; 6:1373-1377.
4.- Sankar D, Rao MR, Sambandam G, Pugalendi KV. Effect of sesame oil on diuretics or Beta-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J Biol Med. 2006;79:19-26.
5.- Vasdev S, Gill V. Antioxidants in the treatment of hypertension. Int J Angiol. 2005;14:60-73
6.- Ukwenya VO, Oluyemi KA, Ashamu E, Saalu C, Oyewo OO, Makanjuola VO. Profertility effects of alcoholic extract of Sesame in male Sprague-Dawley rats . Internet J Nutr Wellness. 2008; 5 (2).
7.- Chang CY, Chen YL, Yang SC, Huang GC, Tsi D, Huang CC, Chen JR, Li JS. Effect of schisandrin B and sesamin mixture on CCl4-induced hepatic oxidative stress in rats. Phytother Res 2008; 23: 251-256.
8.- Oluyemi KA, Omotuyi IO, Jimoh OR, Adesanya OA, Saalu CL, Josiah SJ. Erythropoietic and anti-obesity effects of Garcinia cambogia (bitter kola) in Wistar rats. Biotechnol Appl Biochem. 2007;46:69-72
9.- Aitken RJ, Buckingham D, Harkiss D. Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. J Reprod Fertil 1993; 97:441-450.
10.- Henry MP, Turner TT. Rescue of testicular function following acute experimental torsion. J Urol 1997; 157:340-345.
11.- Southorn PA, Powis G. Free radicals in medicine. I. Chemical nature and physiologic reaction. Mayo Clin Proc. 1988; 63:3881-3889.
12.- Katsuzaki H, Kawasumi M, Kawakishi S, Osawa T. Structure of novel antioxidative lignan glucosides isolated from sesame seed. Biosci. Biotech. Biochem. 1992; 56:2087-2088
13.- Shittu LAJ, Bankole MA, Oguntola JA, Ajala O, Shittu RK, Ogundipe OA, Bankole MN, Ahmed T, Ashiru OA. Sesame leaves intake improve and increase epididymal spermatocytes reserve in adulte male Sprague Dawley rat. Sci Res Essay 2007, 2:319-324.
14.- Kuwada M, Kawashima R, Nakamura K, Kojima H, Hasumi H, Maki J, Suganoc S. Neonatal exposure to endocrine disruptors suppresses juvenile testis weight and steroidogenesis but spermatogenesis is considerably restored during puberty. Biochem Biophys Res Com. 2000; 295:193-197.
15.- Wu HF, Qiao D, Qian LX, Song NH, Feng NH, Hua LX, Zhang W. Congenital agenesis of seminal vesicle. Asian J Androl 2005; 7: 449-452
CORRESPONDENCE:
Oluyemi Kayode Alaba.
Department of Biology, William Paterson University,
NJ, USA.
Email: kayodedanatomist @ yahoo.com
Received October 30, 2009.
Published, December 18, 2009
